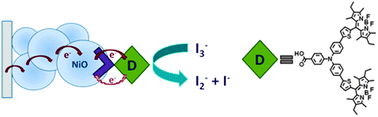Increasing p-type dye sensitised solar cell photovoltages using polyoxometalates
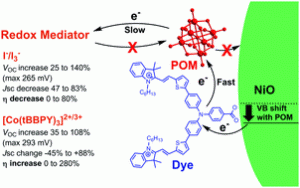
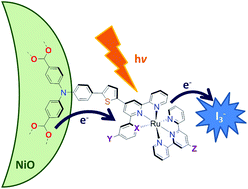
Lindqvist polyoxometalate (POM) additives increase VOC in p-type DSSCs by up to 140%, yielding substantial efficiency gains for poorly matched dyes and redox mediators. For better dye/electrolyte combinations, these gains are typically outweighed by losses in JSC. Charge lifetime and transient IR measurements show that this is due to retardation of both recombination and electron transfer to the mediator, and a positive shift in the NiO valence band edge. The POMs also show their own, limited sensitizing effect.
Charge-transfer dynamics at the dye-semiconductor interface of photocathodes for solar energy applications
This article describes a comparison between the photophysical properties of two charge-transfer dyes adsorbed onto NiO via two different binding moieties. Transient spectroscopy measurements suggest that the structure of the anchoring group affects both the rate of charge recombination between the dye and NiO surface and the rate of dye regeneration by an iodide/triiodide redox couple. This is consistent with the performance of the dyes in p-type dye sensitised solar cells. A key finding was that the recombination rate differed in the presence of the redox couple. These results have important implications on the study of electron transfer at dye|semiconductor interfaces for solar energy applications.
Investigating interfacial electron transfer in dye-sensitized NiO using vibrational spectroscopy.
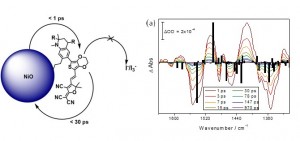
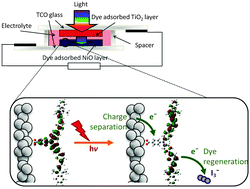
Understanding what influences the formation and lifetime of charge-separated states is key to developing photoelectrochemical devices. This paper describes the use of time-resolved infrared absorption spectroscopy (TRIR) to determine the structure and lifetime of the intermediates formed on photoexcitation of two organic donor–π–acceptor dyes adsorbed to the surface of NiO. The donor and π-linker of both dyes is triphenylamine and thiophene but the acceptors differ, maleonitrile (1) and bodipy (2). Despite their structural similarities, dye 1 outperforms 2 significantly in devices. Strong transient bands in the fingerprint region (1 and 2) and nitrile region (2300–2000 cm−1) for 1 enabled us to monitor the structure of the excited states in solution or adsorbed on NiO (in the absence and presence of electrolyte) and the corresponding kinetics, which are on a ps–ns timescale. The results are consistent with rapid (<1 ps) charge-transfer from NiO to the excited dye (1) to give exclusively the charge-separated state on the timescale of our measurements. Conversely, the TRIR experiments revealed that multiple species are present shortly after excitation of the bodipy chromophore in 2, which is electronically decoupled from the thiophene linker. In solution, excitation first populates the bodipy singlet excited state, followed by charge transfer from the triphenylamine to the bodipy. The presence and short lifetime (τ ≈ 30 ps) of the charge-transfer excited state when 2 is adsorbed on NiO (2|NiO) suggests that charge separation is slower and/or less efficient in 2|NiO than in 1|NiO. This is consistent with the difference in performance between the two dyes in dye-sensitized solar cells and photoelectrochemical water splitting devices. Compared to n-type materials such as TiO2, less is understood regarding electron transfer between dyes and p-type metal oxides such as NiO, but it is evident that fast charge-recombination presents a limit to the performance of photocathodes. This is also a major challenge to photocatalytic systems based on a “Z-scheme”, where the catalysis takes place on a µs–s timescale.
New cyclometalated iridium(III) dye chromophore complexes for p-type dye-sensitised solar cell.
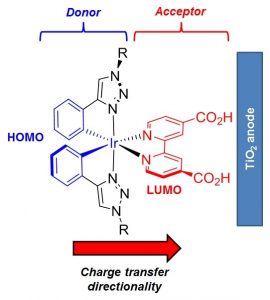
The synthesis of seven iridium complexes [Ir(CˆN)2(NˆN)][PF6] (AS9-15) designed as dyes for p-type DSSC is described. These complexes comprise a 4-(pyrid-2-yl)benzoic acid as the cyclometalating/anchoring ligand with different diimine ligands acting as electron accepting ancillary ligands. DFT analysis together with photophysical investigations reveal how using different π-systems on the ancillary ligand it is possible to tune the absorption spectra of these complexes and to enhance the spatial separation between the HOMO and LUMO. Computational studies demonstrate an ideal HOMO to LUMO charge transfer directionality for the presented [Ir(CˆN)2(NˆN)]+ frameworks, promoting a favourable hole transfer into the NiO cathode valence band upon photoexcitation in p-type DSSC devices. Preliminary tests on NiO-based p-type DSSCs have been carried out confirming the potential use of complexes AS9-15 as a basis for continued development as DSSC chromophores.
Investigation of a new bis(carboxylate)triazole-based anchoring ligand for dye-sensitised solar cell chromophore complexes
A novel anchoring ligand for dye-sensitised solar cell chromophoric complexes, 1-(2,2′-bipyrid-4-yl)-1,2,3-triazole-4,5-dicarboxylic acid (dctzbpy), is described. The new dye complexes [Ru(bpy)2(dctzbpy)][PF6]2 (AS16), [Ir(ppy)2(dctzbpy)][PF6] (AS17) and [Re(dctzbpy)(CO)3Cl] (AS18) were prepared in a two stage procedure with intermediate isolation of their diester analogues, AS16-Et2, AS17-Et2 and AS18-Et2 respectively. Electrochemical analysis of AS16-Et2, AS17-Et2 and AS18-Et2 reveal reduction potentials in the range −1.50 to −1.59 V (vs. Fc+/Fc) which are cathodically shifted with respect to that of the model complex [Ru(bpy)2(dcbH2)]2+ (1) (Ered = −1.34 V, dcbH2 = 2,2′-bipyridyl-4,4′-dicarboxylic acid). This therefore demonstrates that the LUMO of the complex is correctly positioned for favourable electron transfer into the TiO2 conduction band upon photoexcitation. The higher energy LUMOs for AS16 to AS18 and a larger HOMO–LUMO gap result in blue-shifted absorption spectra and hence reduced light harvesting efficiency relative to their dcbH2 analogues. Preliminary tests on TiO2 n-type and NiO p-type DSSCs have been carried out. In the cases of the Ir(III) and Re(I) based dyes AS17 and AS18 these show inferior performance to their dcbH2 analogues. However, the Ru(II) dye AS16 (η = 0.61%) exhibits significantly greater efficiency than 1 (η = 0.1%). In a p-type cell AS16 shows the highest photovoltaic efficiency (η = 0.028%), almost three times that of cells incorporating the benchmark dye coumarin C343.
New cyclometalated iridium(III) dye chromophore complexes for n-type dye-sensitised solar cells
The synthesis of seven iridium complexes where aryl-1,2,3-triazole (Ar-tz) moieties act as cyclometalating ligands with 2,2′-bipyridyl-4,4′-dicarboxylic acid (dcb) as a N^N ancillary/anchoring ligand, is described. The new dye complexes [Ir(Ar-tz)2(dcb)][PF6] (AS1-7) were prepared in a two stage procedure with iridium-chloride dimer isolation. DFT analysis together with photophysical investigations reveal how using different substituents on the phenyl ring, or a different aryl system, lead to the tuning of the absorption and emission properties of these complexes. Computational studies therefore demonstrate an ideal HOMO-LUMO directionality for the [Ir(Ar-tz)2(dcb)]+ framework, promoting a favourable electron transfer into the TiO2 conduction band upon photoexcitation. Preliminary unoptimized tests on TiO2 DSSCs have been carried out which show similar photovoltaic performance to the [Ir(ppy)2(dcb)][PF6] (ppy = 2-phenylpyridine) benchmark.
A resonance Raman study of new pyrrole-anchoring dyes for NiO-sensitized solar cells.
Three dyes for p-type dye-sensitised solar cells containing a novel doubly anchored pyrrole donor group were synthesised and their solar cell performances were evaluated. Dye 1 was comprised of a phenyl–thiophene linker and a maleonitrile acceptor, which has been established as an effective motif in other push–pull dyes. Two boron dipyrromethane analogues, dyes 2 and 3, were made with different linker groups to compare their effect on the behaviour of these dyes adsorbed onto nickel oxide (dye|NiO) under illumination. The photoexcited states of dye|NiO were probed using resonance Raman spectroscopy and compared to dyes anchored using the conventional 4-aminobenzoic acid moiety (P1 and 4). All three components, the anchor, the linker and the acceptor group were found to alter both the electronic structure following excitation and the overall solar cell performance. The bodipy acceptor gave a better performance than the maleonitrile acceptor when the pyrrole anchor was used, which is the opposite of the triphenylamine push–pull dyes. The linker group was found to have a large influence on the short-circuit current and efficiency of the p-type cells constructed.
Do iodine or thiocyanate play a role in p-type dye sensitized solar cells?
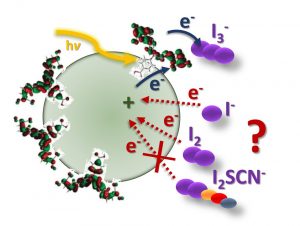
Charge recombination at the NiO/electrolyte interface is one of the performance-limiting factors that prevent p-type dye-sensitized solar cells (p-DSCs) from matching the efficiency of conventional TiO2 devices. This article describes the role of redox mediator species I−, I3−, and I2 in the charge-transfer processes at the NiO/electrolyte interface. NiO appears to catalyze the oxidation of iodide but the addition of guanidinium thiocyanate inhibits recombination by forming a complex with I2. This increases the voltage but decreases the current so the efficiency is only slightly improved. These results suggest that new redox electrolytes will be needed if tandem DSCs are to exceed the efficiency of state-of-the-art TiO2 devices.
Can aliphatic anchoring groups be utilised with dyes for p-type dye sensitized solar cells?
A series of novel laterally anchoring tetrahydroquinoline derivatives have been synthesized and investigated for their use in NiO-based p-type dye-sensitized solar cells. The kinetics of charge injection and recombination at the NiO-dye interface for these dyes have been thoroughly investigated using picosecond transient absorption and time-resolved infrared measurements. It was revealed that despite the anchoring unit being electronically decoupled from the dye structure, charge injection occurred on a sub picosecond timescale. However, rapid recombination was also observed due to the close proximity of the electron acceptor on the dyes to the NiO surface, ultimately limiting the performance of the p-DSCs.
A comprehensive comparison of dye-sensitized NiO photocathodes for solar energy conversion
We investigated a range of different mesoporous NiO electrodes prepared by different research groups and private firms in Europe to determine the parameters which influence good quality photoelectrochemical devices. This benchmarking study aims to solve some of the discrepancies in the literature regarding the performance of p-DSCs due to differences in the quality of the device fabrication. The information obtained will lay the foundation for future photocatalytic systems based on sensitized NiO so that new dyes and catalysts can be tested with a standardized material. The textural and electrochemical properties of the semiconducting material are key to the performance of photocathodes. We found that both commercial and non-commercial NiO gave promising solar cell and water-splitting devices. The NiO samples which had the two highest solar cell efficiency (0.145% and 0.089%) also gave the best overall theoretical H2 conversion.
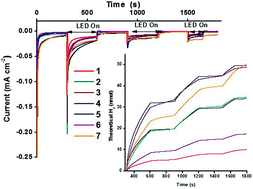
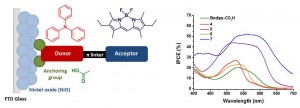
Design and characterisation of bodipy sensitizers for dye-sensitized NiO solar cells
A series of photosensitizers for NiO-based dye-sensitized solar cells is presented. Three model compounds containing a triphenylamine donor appended to a boron dipyrromethene (bodipy) chromophore have been successfully prepared and characterised using emission spectroscopy, electrochemistry and spectroelectrochemistry, to ultimately direct the design of dyes with more complex structures. Carboxylic acid anchoring groups and thiophene spacers were appended to the model compounds to provide five dyes which were adsorbed onto NiO and integrated into dye-sensitized solar cells. Solar cells incorporating the simple Bodipy-CO2H dye were surprisingly promising relative to the more complex dye 4. Cell performances were improved with dyes which had increased electronic communication between the donor and acceptor, achieved by incorporating a less hindered bodipy moiety. Further increases in performances were obtained from dyes which contained a thiophene spacer. Thus, the best performance was obtained for 7 which generated a very promising photocurrent density of 5.87 mA cm−2 and an IPCE of 53%. Spectroelectrochemistry combined with time-resolved transient absorption spectroscopy were used to determine the identity and lifetime of excited state species. Short-lived (ps) transients were recorded for 4, 5 and 7 which are consistent with previous studies. Despite a longer lived (25 ns) charge-separated state for 6/NiO, there was no increase in the photocurrent generated by the corresponding solar cell.
Ni Mg Mixed Metal Oxides for p-Type Dye-Sensitized Solar Cells
Mg Ni mixed metal oxide photocathodes have been prepared by a mixed NiCl2/MgCl2 sol–gel process. The MgO/NiO electrodes have been extensively characterized using physical and electrochemical methods. Dye-sensitized solar cells have been prepared from these films, and the higher concentrations of MgO improved the photovoltage of these devices; however, there was a notable drop in photocurrent with increasing Mg2+. Charge extraction and XPS experiments revealed that the cause of this was a positive shift in the energy of the valence band, which decreased the driving force for electron transfer from the NiO film to the dye and, therefore, the photocurrent. In addition, increasing concentrations of MgO increases the volume of pores between 0.500 and 0.050 μm, while reducing pore volumes in the mesopore range (less than 0.050 μm) and lowering BET surface area from approximately 41 down to 30 m2 g–1. A MgO concentration of 5% was found to strike a balance between the increased photovoltage and decreased photocurrent, possessing a BET surface area of 35 m2 g–1 and a large pore volume in both the meso- and macropore range, which lead to a higher overall power conversion efficiency than NiO alone.
Increased photocurrent in a tandem dye-sensitized solar cell by modifications in push–pull dye-design
Donor–π–acceptor photosensitizers for NiO photocathodes that exhibit a broad spectral response across the visible region are presented. These enabled an increase in the photocurrent density of p-type dye-sensitized solar cells to 8.2 mA cm−2 and a tandem cell to be assembled which generated a photocurrent density of 5.15 mA cm−2.
The ferrocene effect: enhanced electrocatalytic hydrogen production using meso-tetraferrocenyl porphyrin palladium(II) and copper(II) complexes
Copper(II) and palladium(II) meso-tetraferrocenylporphyrins (CuTFcP and PdTFcP) were employed as catalysts for electrochemical proton reduction in DMF using trifluoroacetic acid (TFA) or triethylamine hydrochloride (TEAHCl) as acids. Gas analysis under electrocatalytic conditions at a glassy carbon working electrode confirmed the product as H2. CuTFcP showed catalytic behavior for both TFA and TEAHCl, whereas only TFA worked for PdTFcP. The performance of the two compounds for electrocatalytic hydrogen generation was compared to the analogous copper(II) and palladium(II) meso-tetraphenylporphyrins (CuTPP and PdTPP) under identical conditions. The presence of the ferrocence groups on the porphyrin favourably shift the overpotential to a less negative value by around 200 mV and increases the catalytic rate of hydrogen production in DMF/TFA by an order of magnitude to 6 × 103 s−1. Moreover, while CuTPP is fully inactive in a DMF/TEAHCl mixture, the ferrocene subunits activate theCuTFcP catalyst. Spectroelectrochemistry experiments and DFT calculations were consistent with a catalytic process proceeding via the phlorin anion.
The influence of the preparation method of NiOxphotocathodes on the efficiency of p-type dye-sensitized solar cells

Improving the efficiency of p-type dye-sensitized solar cells (DSCs) is an important part of the development of high performance tandem DSCs. The optimization of the conversion efficiency of p-DSCs could make a considerable contribution in the improvement of solar cells at a molecular level. Nickel oxide is the most widely used material in p-DSCs, due to its ease of preparation, chemical and structural stability, and electrical properties. However, improvement of the quality and conductivity of NiO based photocathodes needs to be achieved to bring further improvements to the solar cell efficiency. The subject of this review is to consider the effect of the preparation of NiO surfaces on their efficiency as photocathodes.
p-type dye sensitized solar cells (p-DSCs) derived from nickel oxide (NiO) photocathodes have been obtained via rapid discharge sintering (RDS) of parent metal oxide nanoparticles deposited onto differently treated substrates utilizing a plasma atmosphere with microwave radiation as heat source. This method produces NiO thin films (0.6<l<6 μm) with mesoporous features and large surface areas as required for efficient dye-loading and high photocurrents. Erythrosine B (ERY) was used to sensitize the oxide in the visible spectrum. We have analyzed and compared the photoelectrochemical performances of the p-DSCs assembled with the various types of NiO samples prepared by RDS techniques with different treatments of the supporting substrate prior to, or during, spray deposition of the NiO nanoparticles. The best photovoltaic performances were obtained when the transparent conducting substrate (TCS) was heated during spraying. We believe that this is because the charge transfer through the NiO film and the charge collection at the TCS/NiO film interface were the most efficient with this sample. To our knowledge, the photovoltaic performances reported here are the best achieved with the commercial dye ERY as sensitizer.
Novel triphenylamine-modified ruthenium(II) terpyridine complexes for nickel oxide-based cathodic dye-sensitized solar cells
A pair of ruthenium based donor-π-chromophore sensitizers (K1 and K2) have been synthesized for use in NiO based p-type dye sensitized solar cells (p-DSCs). The optical and electrochemical properties of these dyes were determined experimentally and interpreted by DFT modelling. NiO p-DSC devices incorporating these dyes gave photocurrents of 2.9 for K1and 2.0 mA cm−2 for K2 (IPCE of 14% and 9%); this is a vast improvement on the photocurrents of p-DSC devices incorporating ‘traditional’ ruthenium sensitizers.
Promoting charge-separation in p-type dye-sensitized solar cells using bodipy
The viability of applying bodipy sensitisers to NiO-based p-type dye-sensitised solar cells (p-DSCs) has been successfully demonstrated. The triphenylamine donor–bodipy acceptor design promotes a long-lived charge-separated state which is difficult to achieve with NiO-based devices. The current was above 3 mA cm−2 and the IPCE was 28%.
Red-Absorbing Cationic Acceptor Dyes for Photocathodes in Tandem Solar Cells

A pair of new donor−π–acceptor dyes that absorb toward the red region of the visible spectrum (CAD 1 and CAD 2) utilizing indolium cationic acceptor units have been synthesized for use in p-type dye-sensitized solar cells (p-DSC). Their optical and electrochemical properties were determined experimentally, including application of ultrafast transient absorption and time-resolved infrared spectroscopies. Our results are supported by computational modeling. NiO-based p-DSCs with CAD 1 and CAD 2 gave short-circuit photocurrent densities of 3.6 and 3.3 mA cm–2, respectively, which are substantially higher than that of any previous red-absorbing p-DSC. These results are a step toward tandem dye-sensitized solar cells that absorb higher-energy photons at the TiO2 anode and lower-energy photons at the NiO cathode. Routes to further improve the efficiency of NiO DSCS are also discussed.
Synthesis and properties of a meso– tris–ferrocene appended zinc(II) porphyrin and a critical evaluation of its dye sensitised solar cell (DSSC) performance
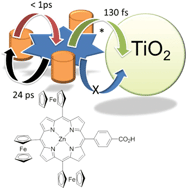
A zinc(II) porphyrin derivative, F3P, was prepared containing a single ferrocene group appended at three of the meso positions. The final meso position contains a benzoic acid unit, and was designed to be an anchoring moiety to a semiconductor surface. The cyclic voltammogram for the porphyrin derivative reveals that the three ferrocene units are oxidised and reduced at E1/2 = +0.04 V vs. Fc+/Fc. Other redox responses are porphyrin-based at +1.1 Vvs. Fc+/Fc (irreversible) and −1.87 V and −2.20 V vs. Fc+/Fc (both quasi-reversible). The room temperature Mössbauer spectrum for F3P comprises a doublet (δ Fe 0.44 mm s−1; ΔEQ 2.35 mm s−1; peak width 0.27 mm s−1). The data are consistent with the ferrocene groups non-interacting with the porphyrin moiety in the ground state. Excitation of the compound in THF with an ultra-short laser pulse produces in less than 1 ps the charge separated state which comprises Fc+˙–porp−˙ and decays back to the ground state in 24 ps. Roughly the same behaviour is observed for the dye absorbed on TiO2, the only slight difference is the appearance of a long-lived component in the decay records. The electron for the porphyrin radical anion does not inject into the conduction band of the TiO2, only the porphyrin excited state participates in any electron injection process. Partly because of this factor the performance of the dye attached to TiO2 in a DSSC device is rather limited (JSC = 0.068 mA cm−2, VOC = 283 mV, FF = 0.42, η = 0.008%).
Dye sensitised solar cells with nickel oxide photocathodes prepared viascalable microwave sintering
Photoactive NiO electrodes for cathodic dye-sensitised solar cells (p-DSCs) have been prepared with thicknesses ranging between 0.4 and 3.0 μm by spray-depositing pre-formed NiOnanoparticles on fluorine-doped tin oxide (FTO) coated glass substrates. The larger thicknesses were obtained in sequential sintering steps using a conventional furnace (CS) and a newly developed rapid discharge sintering (RDS) method. The latter procedure is employed for the first time for the preparation of p-DSCs. In particular, RDS represents a scalable procedure that is based on microwave-assisted plasma formation that allows the production in series of mesoporous NiO electrodes with large surface areas for p-type cell photocathodes. RDS possesses the unique feature of transmitting heat from the bulk of the system towards its outer interfaces with controlled confinement of the heating zone. The use of RDS results in a drastic reduction of processing times with respect to other deposition methods that involve heating/calcination steps with associated reduced costs in terms of energy. P1-dye sensitized NiO electrodes obtained via the RDS procedure have been tested in DSC devices and their performances have been analysed and compared with those of cathodic DSCs derived from CS-deposited samples. The largest conversion efficiencies (0.12%) and incident photon-to-current conversion efficiencies, IPCEs (50%), were obtained with sintered NiO electrodes having thicknesses of ∼1.5–2.0 μm. In all the devices, the photogenerated holes in NiO live significantly longer (τh ∼ 1 s) than have previously been reported for P1-sensitized NiO photocathodes. In addition, P1-sensitised sintered electrodes give rise to relatively high photovoltages (up to 135 mV) when the triiodide–iodide redox couple is used.
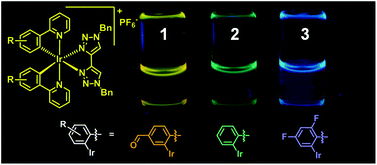
Luminescent biscyclometalated arylpyridine iridium(III) complexes with 4,4′-bi-1,2,3-triazolyl ancillary ligands
The synthesis, characterization and photophysical investigation of complexes of the form [Ir(R-ppy)2(btz)]PF6 (1 to 3) are reported (btz = 1,1′-dibenzyl-4,4′-bi-1,2,3-triazolyl, R-ppy = 4-(pyrid-2-yl)benzaldehyde (1), 2-phenylpyridine (2) and 2-(2,4-difluorophenyl)pyridine (3)). Complexes 1,2 and 3 are luminescent and exhibit structured emission bands with vibronic progressions at 532 & 568 nm (ϕ 0.28%), 476 & 508 nm (ϕ 0.82%) and 454 & 483 nm (ϕ 4.3%) respectively. The structuring of these emission bands is indicative of cyclometalated ligand centred emissive states and is further corroborated by the nearly identical emission spectra for 2 and 3 to previously reported analogous complexes with 4-(pyrid-2-yl)-1,2,3-triazole based ancillary ligands. Computational density functional theory calculations on these complexes show that the LUMOs of 2 and 3 are largely btz-centred but with some cyclometalated pyridine π* character. The LUMO of 1 on the other hand is localized primarily on the cyclometalated ligands. Spin population analysis of the lowest lying triplet excited states for these complexes indicate significant spin population over the iridium centres and the aryl and pyridyl moieties in these complexes with virtually no localization of unpaired electrons over the btz ancillary ligands. This is therefore in agreement with the assignment of the emissive state having largely cyclometalated 3LC character and being independent of the ancillary ligand.
CO2 photoreduction with long-wavelength light: dyads and monomers ofzinc porphyrin and rhenium bipyridine
Photocatalytic CO2 reduction has been studied for two dyads with porphyrin covalently attached to rhenium tricarbonyl bipyridine moieties, and on separate components consisting of [Re(CO)3(Picoline)Bpy]+ and either zinc porphyrin or zinc chlorin. TONs decrease in the order:zinc porphyrin + Re > long spacer dyad > zinc chlorin + Re > short spacer dyad.
Recent advances and future directions to optimize the performances of p-type dye-sensitized solar cells

This review provides a summary of the most important developments in the field of solar cells based on the sensitization of p-type semiconductors, such as NiO, and identifies the future challenges and opportunities to enhance their overall performance. In particular, the main factors responsible for the low open-circuit voltage, short circuit photocurrent and fill factor are discussed in detail.
Role of the Triiodide/Iodide Redox Couple in Dye Regeneration in p-Type Dye-Sensitized Solar Cells

A series of perylene dyes with different optical and electronic properties have been used as photosensitizers in NiO-based p-type dye-sensitized solar cells. A key target is to develop dyes that absorb light in the red to near-infrared region of the solar spectrum in order to match photoanodes optically in tandem devices; however, the photocurrent produced was found to decrease dramatically as the absorption maxima of the dye used was varied from 517 to 565 nm and varied strongly with the electrolyte solvent (acetonitrile, propionitrile, or propylene carbonate). To determine the limitations of the energy properties of the dye molecules and to provide guidelines for future sensitizer design, we have determined the redox potentials of the diiodide radical intermediate involved in the charge-transfer reactions in different solvents using photomodulated voltammetry. E°(I3–/I2•–) (V vs Fe(Cp)2+/0) = −0.64 for propylene carbonate, −0.82 for acetonitrile, and −0.87 for propionitrile. Inefficient regeneration of the sensitizer appears to be the efficiency-limiting step in the device, and the values presented here will be used to design more efficient dyes, with more cathodic reduction potentials, for photocathodes in tandem dye-sensitized solar cells.
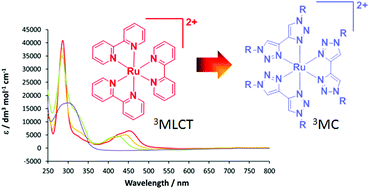
Synthesis, characterisation and theoretical study of ruthenium 4,4′-bi-1,2,3-triazolyl complexes: fundamental switching of the nature of S1 and T1 states from MLCT to MC
The series of complexes [Ru(bpy)3−n(btz)n][PF6]2 (bpy = 2,2′-bipyridyl, btz = 1,1′-dibenzyl-4,4′-bi-1,2,3-triazolyl, 2n = 1, 3n = 2, 4n = 3) have been prepared and characterised, and the photophysical and electronic effects imparted by the btz ligand were investigated. Complexes 2and 3 exhibit MLCT absorption bands at 425 and 446 nm respectively showing a progressive blue-shift in the absorption on increasing the btz ligand content when compared to [Ru(bpy)3][Cl]2 (1). Complex 4 exhibits a heavily blue-shifted absorption spectrum with respect to those of1–3, indicating that the LUMO of the latter are bpy-centred with little or no btz contribution whereas that of 4 is necessarily btz-centred. DFT calculations on analogous complexes 1′–4′ (in which the benzyl substituents are replaced by methyl) show that the HOMO–LUMO gap increases by 0.3 eV from 1′–3′ through destabilisation of the LUMO with respect to the HOMO. The HOMO–LUMO gap of 4′ increases by 0.98 eV compared to that of 3′ due to significant destabilisation of the LUMO. Examination of TDDFT data show that the S1 states of 1′–3′ are1MLCT in character whereas that of 4′ is 1MC. The optimisation of the T1 state of 4′ leads to the elongation of two mutually trans Ru–N bonds to yield [Ru(κ2-btz)(κ1-btz)2]2+, confirming the 3MC character. Thus, replacement of bpy by btz leads to a fundamental change in the ordering of excited states such that the nature of the lowest energy excited state changes from MLCT in nature to MC.
Cobalt Polypyridyl-Based Electrolytes for p-Type Dye-Sensitized Solar Cells

A series of polypyridyl cobalt complexes with different substituents was applied as redox mediators in p-type dye-sensitized solar cells (p-DSCs), consisting of mesoporous NiO sensitized with a perylenemonoimide−naphthalenediimide (PMI-NDI) dyad. The photocurrent and photovoltages of the devices were found to depend on the steric bulk of the redox species rather than their electrochemical potential. Bulky substituents were found to slow the detrimental charge recombination reactions between holes in the NiO semiconductor and the reduced form of the redox couple. The open-circuit potential (VOC) of each of the devices was superior to the equivalent PMI-NDI-sensitized p-DSCs containing the triiodide/iodide redox couple.
Photomodulated Voltammetry of Iodide/Triiodide Redox Electrolytes and Its Relevance to Dye-Sensitized Solar Cells

Photomodulated voltammetry was used to determine the redox potentials of the diiodide radical (I2–•) in water, acetonitrile, and 3-methoxypropionitrile. Iodide/triiodide redox electrolytes were exposed to modulated blue light, resulting in I2–• generation. Using transparent fluorine-doped tinoxide (FTO) electrodes, two modulated photocurrent waves could be discerned in the voltammogram, from which the formal potentials for oxidation and reduction reactions of the diiodide radical were determined. E0′(I2–•/I–) was found to be +0.79 and +1.04 V versus NHE in acetonitrile and water, respectively. These values give guidelines for E0′(D+/D) required for efficient regeneration of dyes used in dye-sensitized solar cells
Synthesis, photophysical and photovoltaic investigations of acceptor-functionalized perylene monoimide dyes for nickel oxide p-type dye-sensitized solar cells
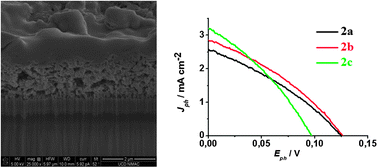
We report on the synthesis, electrochemical, photophysical, and photovoltaic properties of a series of three organic dyads comprising a perylene monoimide (PMI) dye connected to a naphthalene diimide (NDI) or a fullerene (C60) for application in dye-sensitized solar cells (DSCs) with nanocrystalline NiO electrodes. It was found that the secondary electron acceptor(NDI or C60) in all the three dyads extends the charge separated state lifetime by about five orders of magnitude compared to the respective parent PMI dye. Nanosecond pump–probe experiments of the NiO/dyads in the presence of the electrolyte show that the reduction of triiodide by the secondary electron acceptor is slow in all the dyads, which we ascribe to a weak driving force for this reaction. This reaction is significantly faster with the cobalt electrolyte (tris(4,4′-di-tert-butyl-2,2′-bipyridine)cobalt(II/III)), whose driving force is larger; however, its reaction with the reduced dyads is still rather slow. We demonstrate that the larger photovoltage observed with the cobalt electrolyte (VOC = 285 mV) relative to the iodide electrolyte (VOC = 120 mV) is due to a decrease in the dark current for the former owing to slower interfacial electron transfer of the reduced mediator with the injected holes into the NiO electrode. In terms of photovoltaic performances, the most efficient dyad is the system in which the NDI is directly connected to the PMI (η = 0.14% under AM 1.5 with the cobalt electrolyte), but the dyad containing the fullerene acceptor exhibits the highest IPCE and the highest short circuit current density (IPCE = 57%, JSC = 1.88 mA cm−2) with the iodide electrolyte. The latter performances are attributed to the slightly stronger reducing power of C60 relative to NDI, which favours the reduction of the mediator in the electrolyte.
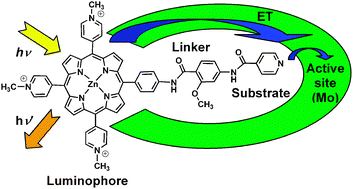
Design and synthesis of water soluble (metallo)porphyrins with pendant arms: studies of binding to xanthine oxidase
The tethering of a substrate analogue to a covalently attached luminophore may give rise to aprobe for reporting enzyme activity spectrofluorometrically. The overall design incorporates awater-soluble porphyrin luminophore, a substrate analogue and a planar conjugated bridge of the appropriate length designed to bind in the substrate-access channel of xanthine oxidase (XO). 5,10,15-Tris(N-methyl-4-pyridiniumyl)-20-phenyl porphyrins was functionalised at the 4-position of the phenyl group with amide-linked 2-methoxy-benzamide groups, [(2-methoxy-4-amino-phenylcarbonyl)-amino] or [(2-methoxy-4-[(pyridine-4-carbonyl)-amino]-phenylcarbonyl)-amino]. The products were isolated as free base or zinc porphyrins with chloride orhexafluorophosphate counterions and characterised by optical emission and absorption in addition to other spectroscopic methods. Binding studies of bovine XO to the zinc porphyrintrichloride derivatives with the above linkers were used to determine the IC50 of 81 and 113 μM and Ki values of 4.6 and 6.5 μM, respectively. Furthermore, addition of XO to an aqueous solution of the products caused quenching of the porphyrin emission and a red shift in theabsorption spectrum.
Design of Organic Dyes and Cobalt Polypyridine Redox Mediators for High-Efficiency Dye-Sensitized Solar Cells

Dye-sensitized solar cells (DSCs) with cobalt-based mediators with efficiencies surpassing the record for DSCs with iodide-free electrolytes were developed by selecting a suitable combination of a cobalt polypyridine complex and an organic sensitizer. The effect of the steric properties of two triphenylamine-based organic sensitizers and a series of cobalt polypyridine redox mediators on the overall device performance in DSCs as well as on transport and recombination processes in these devices was compared. The recombination and mass-transport limitations that, previously, have been found to limit the performance of these mediators were avoided by matching the properties of the dye and the cobalt redox mediator. Organic dyes with higher extinction coefficients than the standard ruthenium sensitizers were employed in DSCs in combination with outer-sphere redox mediators, enabling thinner TiO2 films to be used. Recombination was reduced further by introducing insulating butoxyl chains on the dye rather than on the cobalt redox mediator, enabling redox couples with higher diffusion coefficients and more suitable redox potential to be used, simultaneously improving the photocurrent and photovoltage of the device. Optimization of DSCs sensitized with a triphenylamine-based organic dye in combination with tris(2,2′-bipyridyl)cobalt(II/III) yielded solar cells with overall conversion efficiencies of 6.7% and open-circuit potentials of more than 0.9 V under 1000 W m−2 AM1.5 G illumination. Excellent performance was also found under low light intensity indoor conditions.
Double-Layered NiO Photocathodes for p-Type DSSCs with Record IPCE

A way to achieve a high-efficiency dye-sensitized solar cell is to combine an n-type TiO2-based photoanode with a p-type photocathode in a tandem configuration. The development of an efficient photocathode is, at present, the key target. We have optimized the NiO, I3−/I− p-DSSC system to obtain record photocurrent, giving 64% incident photon-to-current conversion efficiency (IPCE) and 5.48 mAcm−2JSC.
Synthesis and Mechanistic Studies of Organic Chromophores with Different Energy Levels for p-Type Dye-Sensitized Solar Cells

A series of donor−π−acceptor dyes with different electron-withdrawing groups were designed and synthesized for p-type dye-sensitized solar cells. The modification of dye structures shows significant influence on the photophysical, electrochemical, and photovoltaic performance of the dyes. DSSCs based on these dyes show maximum 63% and minimum 6% of incident monochromatic photon-to-current conversion efficiencies. The two dyes with the highest (P1) and lowest (P3) efficiencies were studied by femtosecond transient absorption spectroscopy, which shows a fast injection rate of more than (250 fs)−1 for both dyes. Such fast injection corresponds to more than 90% injection efficiency. The photoinduced absorption spectroscopy study of sensitized NiO films in the presence of electrolyte showed poor regeneration of P3 due to an insufficient driving force. This, together with aggregation of the dye on the NiO film, explained the poor solar cell performance.
A p-Type NiO-Based Dye-Sensitized Solar Cell with an Open-Circuit Voltage of 0.35 V

In tandem: Employing a molecular dyad and a cobalt-based electrolyte gives a threefold-increase in open-circuit voltage (VOC) for a p-type NiO device (VOC=0.35 V), and a fourfold better energy conversion efficiency. Incorporating these improvements in a TiO2/NiO tandem dye-sensitized solar cell (TDSC), results in a TDSC with a VOC=0.91 V (see figure; CB=conductance band, VB= valence band).
Dye Regeneration by Spiro-MeOTAD in Solid State Dye-Sensitized Solar Cells Studied by Photoinduced Absorption Spectroscopy and Spectroelectrochemistry

Photoinduced absorption (PIA) spectroscopy is presented as a tool for the systematic study of dye regeneration and pore filling in solid state dye-sensitized solar cells (DSC). Oxidation potentials and extinction coefficients for oxidized species of the perylene dye, ID28, on TiO2and of the hole conductor, 2,2′7,7′-tetrakis-(N,N-di-p-methoxyphenyl-amine)-9,9′-spirobifluorene (spiro-MeOTAD), were determined by spectroelectrochemistry. The onset of oxidation of a solid film of spiro-MeOTAD was found to be 0.15 V versus Fc/Fc+ and extinction coefficients of spiro-MeOTAD+ were found to be 33 000 M−1 cm−1 at 507 nm and 8500 M−1cm−1 at 690 nm. Electrons in TiO2 films were shown to alter the ground-state absorption spectra of ID28 attached to TiO2. PIA measurements indicated a good contact between ID28 and spiro-MeOTAD for different spiro-MeOTAD concentrations for both 2- and 6-μm thick TiO2 films. We discuss the possibility of estimating the quality of pore filling from the positions of absorption peaks. Results suggested that with a spiro-MeOTAD concentration of 300 mg mL−1 in chlorobenzene, a uniform distribution of spiro-MeOTAD in the pores of the 6-μm thick TiO2 film could be achieved.