Research in the Hopkinson Group spans several areas of organic synthesis with an overall focus on developing new practical reaction methodologies. Particular areas of interest include organofluorine chemistry, photochemistry and organocatalysis.
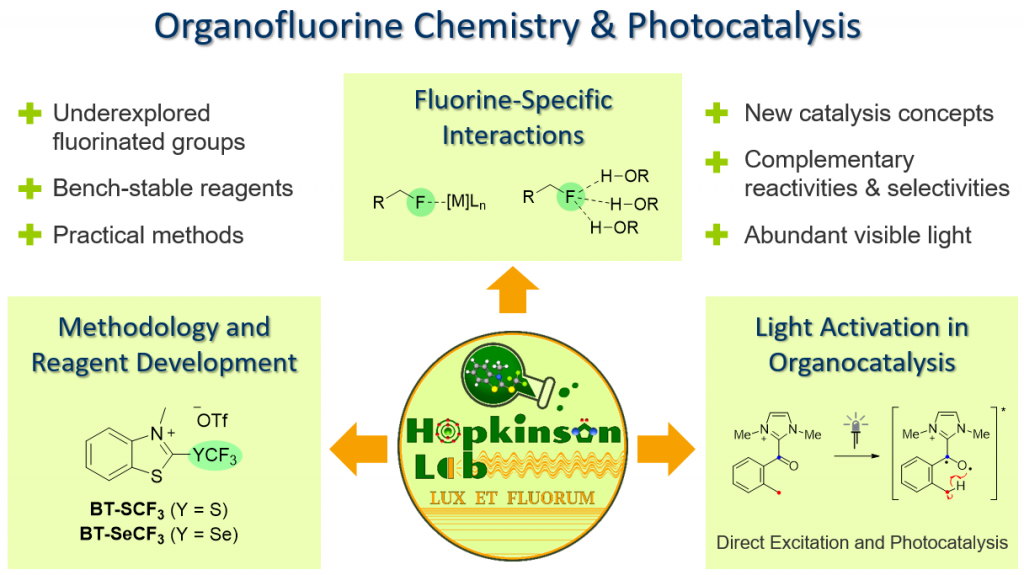
Organofluorine Chemistry
The incorporation of fluorine or fluorine-containing groups into organic molecules can have a dramatic impact on their chemical and biological properties. In particular, fluorinated pharmaceuticals often exhibit improved potency compared to their non-fluorinated analogues and are more metabolically stable. The comparative lack of fluorine-containing natural products means that access to these compounds relies heavily on synthetic organic chemistry and new methods that offer improved efficiency and selectivity or even allow for the preparation of unprecedented classes of organofluorine compound are highly desired.
Our research is centred on the design and application of new methodologies for the synthesis of fluorine-containing organic molecules relevant as pharmaceuticals and agrochemicals. One project line concerns the synthesis, development and application of new widely applicable reagents for installing medicinally relevant fluorinated groups such as the CF3, OCF3, SCF3 and SF5 moieties. For example, in recent work, we have introduced BT-Reagents as a new class of solid reagents that enable the deoxygenative synthesis of (fluoroalkyl)thio- and seleno-substituted compounds directly from widely available alcohols and carboxylic acids.
In other projects we exploit visible light as a tool for enabling the installation and manipulation of fluorine and fluorine-containing functional groups. For example, we have developed a new radical method for preparing valuable trifluoromethoxylated compounds using bis(trifluoromethyl)peroxide (BTMP) as a practical OCF3 radical source. Initiated by our involvement in the DFG-supported Collaborative Research Centre (SFB 1349) “Fluorine-Specific Interactions”, we also investigate selective C−F activation reactions which exploit the unique interactions between fluorine atoms and Lewis acids or hydrogen bond donors. These processes enable the synthesis of valuable partially fluorinated compounds from widely available perfluorinated feedstocks.
Light Activation in Organocatalysis
Alongside our work on organofluorine chemistry, we also develop new reaction methodologies involving photoactivation. As most compounds do not themselves absorb light in the visible part of the spectrum, we take advantage of photocatalysis strategies wherein photocatalysts such as organic dyes or transition metal polypyridyl complexes like [Ru(bpy)3]2+ (bpy = 2,2ʹ-bipyridine) transfer energy either directly or via electron transfer to the substrates of interest. These processes, not only allow abundant and sustainable visible light to be used as an energy source in organic synthesis, but can also lead to unprecedented and exotic reactivity by giving access to reactive radical or ionic intermediates under remarkably mild conditions (typically room temperature using household desk lamps or LEDs).
In one project line, we are focused on the development of new methodologies where light activation is combined with N-heterocyclic carbene (NHC) organocatalysis. Using such approaches, reactivities and selectivities that are complementary to existing NHC-catalysed processes can be obtained while the wide availability of chiral organocatalysts opens up opportunities for conducting otherwise challenging enantioselective photochemical reactions. In recent work, we have introduced Photo-NHC catalysis as a strategy for expanding the scope of carbonyl photochemical reactions to substrates at the carboxylic acid oxidation level. In this process, the NHC organocatalyst can be considered a “transient aryl group” that temporarily changes the absorption characteristics and excited state properties of the carbonyl function during the catalytic cycle.

