We’ve made a few small changes to the Achiever system, please read on for more details and notice of an upcoming change which will affect all users.
New Sample field
We’ve added ‘Visit Number’ to the sample screen, which is useful for teams who wish to assign samples to a particular visit or session without the overhead of a custom clinical data form.
Sample grid refresh
When inserting samples in bulk, the samples grid would not refresh by default to show the new samples. This could be confusing and now the sample grid will automatically refresh.
Consent Restrictions
A new field has been added to the Informed Consent form to capture restrictions which are not catered for by the existing opt-out fields. Entering data into this free-text field will result in a warning being displayed on sample records linked to the participant, just like it would with a specific opt-out.
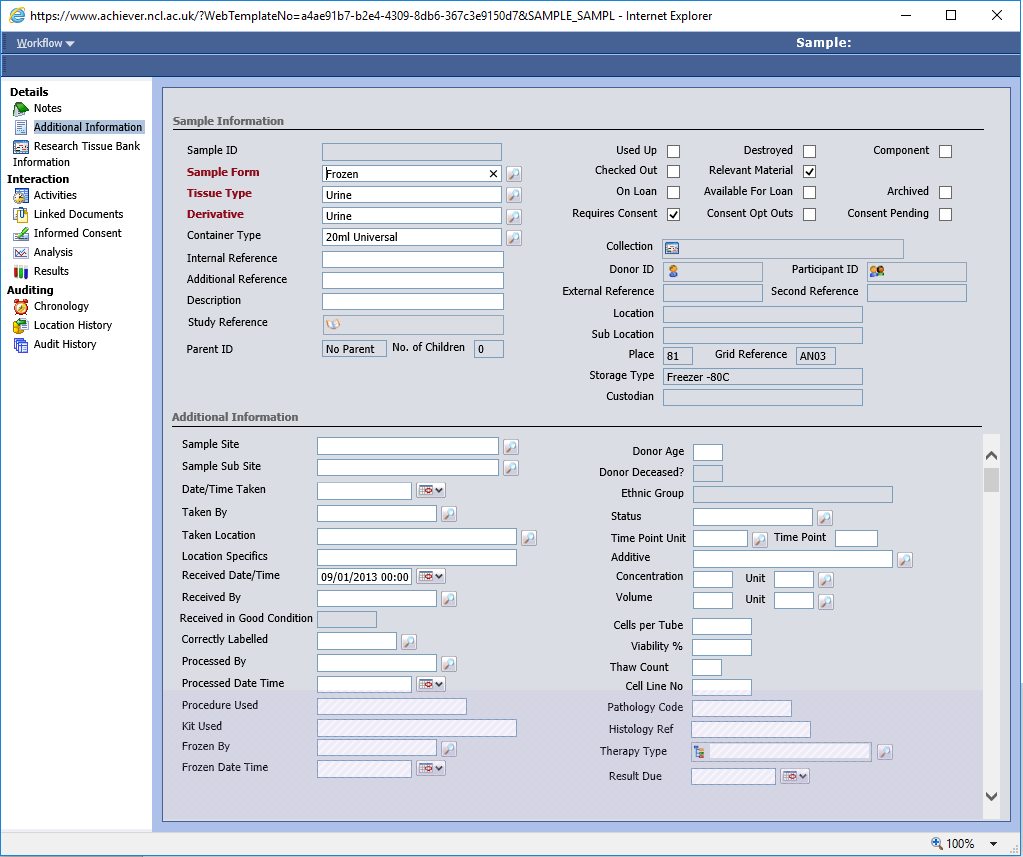
Sample screen cleanup
You may have noticed that sample-level fields are scattered across both the main Sample Information area (top half) and the Additional Information area (bottom half) of the Sample screen. It’s not always very clear why some fields sit on the top, and some below so we are planning on moving a number of fields around to make this forms easier to use. The intention is to keep a small number of key fields on the top screen, with all other sample fields being part of the Additional Information form. An example of how it may look is shown below (click for full-size):
We are also taking the opportunity to remove unused fields ‘CTR Code’, ‘Tumour Type’, ‘Growth Properties’ and ‘Growth Medium’ fields. Other changes are possible, and we are planning on introducing a Barcode field, for users who use pre-barcoded containers or receive pre-barcoded samples. Keep a lookout for these changes towards the end of the week.
If you have any questions or comments please let the team know.