Citation: Lee JY, Dong SH, Ng KW, Goh CF. Assessing the integrity and mechanical properties of commercial microneedles: innovation or fad? Drug Deliv Transl Res. 2025. doi: 10.1007/s13346-025-01888-8
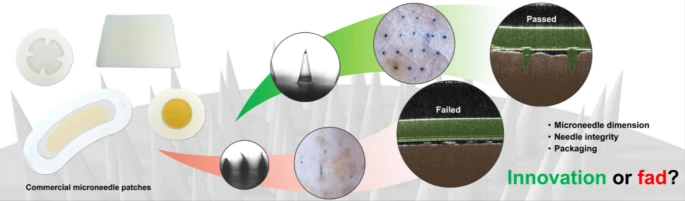
In this collaborative paper—our second with the Malaysian team led by Dr Choon Fu Goh—we examine some commercially available cosmetic products and ask what lessons we can learn from them to enhance pharmaceutical microneedle product translation and commercialisation.
We have known, for a long time, that the regulatory hurdles for pharmaceutical products are much greater than those for cosmetic products. Still, it’s interesting to see how cosmetic microneedle products have surged years (if not decades) ahead of their pharmaceutical counterparts, particularly in the Asian market. A low regulatory hurdle could spur innovation, but it could equally grow fad. How can one tell which it is? We examined a selection of commercially available cosmetic microneedle products to find out, and report our findings in this paper.
This has been an interesting paper to work on. I have admired Goh’s tenacity collecting microneedle patches from pharmacies on his various international trips across Asia for this study. Last year, I hosted him in Newcastle to conduct parts of the study, including some microscopy work and the optical coherence tomography (OCT) analysis on microneedle penetration in ex vivo pig skin. It’s rewarding to see those efforts pay off.
