
We went out for a meal with some current and former lab members. It was great to catch up over some lovely food and drink, and learn that everyone was doing well. We missed a few people who couldn’t join us, but we’ll surely keep in touch!

Dr Keng Wooi Ng @ Newcastle University

We went out for a meal with some current and former lab members. It was great to catch up over some lovely food and drink, and learn that everyone was doing well. We missed a few people who couldn’t join us, but we’ll surely keep in touch!
Ng KW, Archbold L, Lau WM. Building Otto: An open-source Franz diffusion cell autosampler for automating in vitro skin permeation studies. HardwareX. 2026;25:e00735. doi: 10.1016/j.ohx.2025.e00735
We have recently published a paper in HardwareX describing the design and construction of Otto, an open-source autosampler robot for Franz diffusion cell experiments. Otto is built using a desktop 3D printer as a gantry, a small number of custom 3D-printed parts, and commonly available laboratory consumables. It is designed to automate sampling and can collect up to 100 samples per run.
The system uses a Creality Ender 3 Pro 3D printer for motion control, with add-on components printed in-house on Prusa Research printers. The aim of the project was to develop a low-cost, accessible solution for automating repetitive sampling tasks in skin permeation studies, without reliance on proprietary hardware.
Otto has previously been validated. In a recent hydrogel study, Otto was used to collect every sample in a 72-hour skin permeation experiment, operating fully unattended throughout. The new HardwareX paper brings together the design rationale, build instructions and practical considerations needed for others to construct and use the system.
The paper, which is now available, provides a step-by-step guide to building Otto and is intended to support reproducibility and reuse by other laboratories. The 3D models and design files are openly available, and the models are also hosted on Printables.
Alongside the hardware, a companion application called OttoMate has been developed to generate the G-code used to control the system via a graphical user interface. The software is under active development and is available on GitHub.
A series of videos demonstrating different aspects of Otto’s operation, including assembly and sampling, is also available via a YouTube playlist.
This work was carried out by our team at Newcastle University, with contributions from Liam Archbold and Dr Wing Man Lau. Otto has been open-sourced in the hope that others will find it useful and adapt it for their own applications, and we are open to collaborations.
We would also like to acknowledge the EPSRC for funding this work.
Stephenson H, Lim A, Dupuy R, Brun S, Armstrong A, Okunola N, Li NA, Hilkens CMU, Novakovic K, Hills S, Ng KW, Al Musaimi O. Elastin-derived peptide hydrogels for sustained dermal delivery of tetrapeptide-21. Int J Pharm, 2026;689:126490. https://doi.org/10.1016/j.ijpharm.2025.126490
Last year, we published a paper with Othman and co-workers about a series of elastin-derived peptide (EDPs) capable of self-assembling into hydrogels. We envisaged that the hydrogels could be used to deliver drugs into the skin.
Our follow-on paper, published today, confirms that EDPs can indeed not only deliver drugs to the skin, but sustain their release.
In this paper, we rationally designed and evaluated a series of self-assembling hydrogels made from synthetic peptides. These peptides have been derived from elastin, the natural protein that gives our skin its elasticity. We encapsulated tetrapeptide-21, a collagen-stimulating peptide, in the hydrogel and successfully delivered it across the skin, demonstrating sustained release for at least 72 hours in vitro.
Collagen production is, of course, critical to solving many skin-related problems, from wrinkle reduction to wound healing. We think this hydrogel technology will find great use in dermatology and aesthetic medicine.
This is the first paper in which we have reported the skin-permeabilising effects of a fractional ablative laser and a motorised microneedle device, in collaboration with Lynton.
This is also the first paper where Franz diffusion cell (FDC) sampling in the skin permeation experiments was fully automated with Otto in a real-world experiment. Otto is an open-source FDC autosampler robot we have developed in-house from a cartesian 3D printer, some 3D-printed parts and common laboratory consumables. We have previously published its design files and validation data.
This has been a great collaboration with many colleagues. We are grateful for the opportunity to collaborate and thank everyone who has contributed to this work.
Featured image reproduced from Stephenson et al., 2025 under a CC BY 4.0 licence.
Yay! Rach came to attend her PhD graduation ceremony today and paid us a visit. I think she was reasonably impressed by the new capabilities we’ve acquired and progress made since she finished her PhD research with us.
Many congratulations, Dr. Rachael Dixon!


What if we could detect or even predict skin cancer with a simple skin swab, before a tumour is even visible?
Our team at Newcastle University is developing this technology, and we’re looking for a passionate PhD student to join us. This is a fully-funded project that sits at the exciting intersection of biology, engineering, and real-world patient impact.
Skin cancer is one of the UK’s most common cancers. We’re tackling this by pioneering a gentle, minimally invasive technique that collects molecular information from beneath the skin’s surface. This ‘microsampling’ approach could one day make community screening for skin cancer a simple and accessible reality.
You will be at the heart of this innovation, working to optimise the technique, discover key biomarkers, and test its use in a clinical setting.
This project is a close collaboration with Professor Mark Birch-Machin (Professor of Molecular Dermatology), Dr Andrew Porter of the Newcastle University Protein and Proteome Analysis (NUPPA) facility, and Dr Samantha Hills (Clinical Director at Lynton, the UK’s largest manufacturer of aesthetic, surgical and conservation lasers).
This isn’t a standard lab-based PhD. It’s a prestigious MRC DiMeN iCASE studentship, which means you’ll be co-developing this technology with our industrial partner, Lynton.
Your experience will include:
We’re looking for a curious and motivated student with a background in life sciences or biomedical engineering. If you’re driven by the prospect of seeing your work directly improve patient lives and want a PhD that prepares you for a career at the cutting edge of health tech, we want to hear from you.
Find all the details and apply via the official advertisement on FindAPhD.com and DiMeN website. Apply by 4 December 2025, 1 pm (GMT). Enquiries welcome.
Please share this with anyone in your network who might be the perfect fit for this project!
Ng KW. Design files for Otto, the Franz diffusion cell autosampling robot, Mendeley Data, V1, 2025. https://doi.org/10.17632/cvc9vxjgn9.1
We have open-sourced the design files for Otto, the Franz diffusion cell autosampler that we built to automate skin permeation studies. This means we’re sharing the 3D-printable models with the world, for free. Anyone may print, modify and share these files with others — with attribution. The files are released under the CC BY 4.0 licence.
Download the files using either link: Mendeley Data | Printables
Here’s the LinkedIn post announcing the release.
Here’s a video showing Otto in action:
I was recently invited by Dr. Ding Gongtao, Associate Professor at Northwest Minzu University, China, to visit his lab and the Biomedical Research Center. Dr. Ding specializes in biomaterials research, and I was deeply impressed by both the quality of the facilities and the breadth of projects his team is pursuing.
During my visit, I had the chance to meet his research group, exchange ideas, and explore opportunities for collaboration. The discussions were engaging and highlighted exciting possibilities for joint work in the future.
One of the highlights of this trip was delivering my first ever research seminar in Mandarin. Preparing for it was quite a challenge, as I had to translate all scientific terminology from English into Chinese. With some help from Baidu Translate, I managed to put it together. The experience was both challenging and rewarding, and it’s something I would gladly do again.
Beyond the seminar, I learnt a great deal from Dr. Ding and his colleagues, not just about research, but also about perspectives shaped by different academic and cultural contexts. This exchange was truly enriching, and I am grateful for the warm hospitality I received throughout my stay.
I look forward to continuing this dialogue and building many fruitful collaborations with Dr. Ding and his team.






Abdelghany TM, Vo N, Vukajlovic D, Smith E, Wong JZ, Jackson E, Hilkens CMU, Lau WM, Ng KW, Novakovic K. Engineering and in vitro evaluation of semi-dissolving, hydrogel-forming polymeric microneedles for sustained-release drug delivery. Int J Pharm. 2025:125932. https://doi.org/10.1016/j.ijpharm.2025.125932

In our latest paper, we describe a microneedle formulation that utilises two polymeric domains: a soluble one and an insoluble one. The insoluble domain is chemically crosslinked and traps the soluble polymer, along with the drug, within it. This combination creates a microneedle array patch that can release a drug for over 2 months.
It can contain a significantly larger dose than microneedles where the drug is contained within the microneedle tips only (e.g., detachable microneedles). The drug reservoir in the backplate makes this possible to support extended release. It uses one-pot synthesis, a mild hydroalcoholic solvent system and mild temperatures to aid manufacturability and drug stability.
For the first time, we were able to see, on video, how the microneedles released the drug and swell as they hydrated. These videos are buried in the supplementary files for the paper, but I thought it worthy of sharing more widely here:
Videos from Abdelghany et al. (2025). Reused under a Creative Commons licence.
We would like to thanks everyone who’s contributed to this paper. Big thanks to the EPSRC and Innovate UK for funding this work.
Citation: Lee JY, Dong SH, Ng KW, Goh CF. Assessing the integrity and mechanical properties of commercial microneedles: innovation or fad? Drug Deliv Transl Res. 2025. doi: 10.1007/s13346-025-01888-8
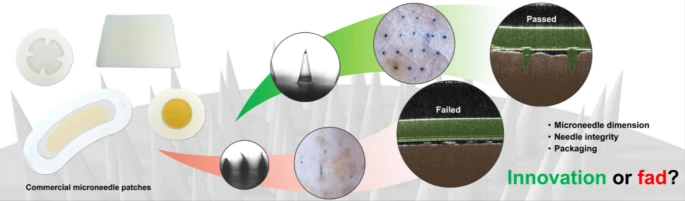
In this collaborative paper—our second with the Malaysian team led by Dr Choon Fu Goh—we examine some commercially available cosmetic products and ask what lessons we can learn from them to enhance pharmaceutical microneedle product translation and commercialisation.
We have known, for a long time, that the regulatory hurdles for pharmaceutical products are much greater than those for cosmetic products. Still, it’s interesting to see how cosmetic microneedle products have surged years (if not decades) ahead of their pharmaceutical counterparts, particularly in the Asian market. A low regulatory hurdle could spur innovation, but it could equally grow fad. How can one tell which it is? We examined a selection of commercially available cosmetic microneedle products to find out, and report our findings in this paper.
This has been an interesting paper to work on. I have admired Goh’s tenacity collecting microneedle patches from pharmacies on his various international trips across Asia for this study. Last year, I hosted him in Newcastle to conduct parts of the study, including some microscopy work and the optical coherence tomography (OCT) analysis on microneedle penetration in ex vivo pig skin. It’s rewarding to see those efforts pay off.
Citation: Chan HKY, Archbold L, Lau WM, Ng KW. Validating Otto: a Franz diffusion cell autosampler to automate in vitro permeation studies, Journal of Pharmaceutical Sciences, 2025:103837. https://doi.org/10.1016/j.xphs.2025.103837
We have a new paper out. This one is close to my heart because I personally spent many hands-on hours developing Otto.
Who’s Otto?
Otto is a Franz diffusion cell (FDC) autosampler robot. It replaces manual sampling and refilling of FDCs in a skin (and cornea, mucosal membrane, etc.) permeation experiment. Those who have worked with FDCs before would know how fiddly, time-consuming and labour-intensive that is. It’s a job most suited for a robot.
But Otto is about more than us trying to avoid menial labour. It’s about the quality of the science, too.
Let me rephrase that — it’s primarily about the quality of the science.
For a long time, we have noted many skin drug absorption studies that include unusually large sampling gaps of ≥16 hours, presumably because the researchers were unable to collect samples outside normal working hours. This sampling gap could allow the drug to accumulate in the FDC receptor chamber and, consequently, underestimate drug absorption due to sink condition being violated. We have faced similar logistical challenges ourselves as local rules prevent some researchers from working out of normal working hours. A FDC autosampler would solve these challenges, but we have not been able to afford any of the few commercial FDC automation systems available. When COVID-19 hit, and lab access was further restricted, we finally found the impetus and time to build the FDC autosampler we had always needed, for less than £500, and retrofitted it to our existing FDCs.
Thus, Otto was born.

We have spent the last 2 years validating Otto’s performance. In this paper, we demonstrate that the sampling gap indeed led to violation of sink condition and underestimation of drug absorption. We further show that Otto improved data quality by avoiding the sampling gap. We have benchmarked Otto’s precision and accuracy against a trained researcher. We are pleased to report that it outperforms the researcher on both counts.
Otto is better than the commercial offerings in many ways. It is built on open-source technologies, using inexpensive consumables and 3D-printed custom parts, and is therefore fully customisable. It has a small footprint of just 50 cm × 46 cm. It can be retrofitted to generic FDCs and can collect up to 100 samples per experiment, fully unattended. The samples are collected directly into high-performance liquid chromatography (HPLC) autosampler vials, so it integrates seamlessly with downstream analysis without any further liquid handling, nor modification to the FDCs or analytical equipment.
Logistical, human resource and financial constraints continue to grip many research organisations long after COVID-19 restrictions have ended. Otto should prove itself a valuable asset in many research labs seeking to retrofit an automation solution to their existing FDCs.
The build instructions for Otto are too extensive to include in this paper, so we will be publishing them separately.