One of the newest recruits to ICaMB is Professor Bert van den Berg, who arrived here in December 2012. Bert is already off to a great start having been awarded a Royal Society Wolfson Research Merit Award in April. Here we have asked him to tell us why he decided to join ICaMB and the research that lead up to this prestigious award.
By Bert van den Berg

Bert, looking thrilled
I joined ICaMB in January, coming from the University of Massachusetts Medical School in Worcester, where I was a tenured faculty member in the Program in Molecular Medicine. While I had a great and productive time in this department, after eight years I felt increasingly isolated academically and started to look for another position. ICaMB seemed a great fit for my research interests, with a large number of scientists interested in bacterial biochemistry and cell biology. Since ICaMB was also looking to strengthen its efforts in structural biology, the decision to cross the pond and join ICaMB wasn’t a very hard one. I am happy to be here, and I hope and expect that my expertise in membrane protein structural biology will also be a benefit for the faculty within ICaMB and will lead to successful collaborations.
My lab has been studying protein channels (see below) for about nine years. Determining structures is really the only way to obtain deep insights into protein function. In addition, seeing a new protein structure for the first time is often an “aha!” moment and, at least for me, the closest thing to a true discovery in modern science. In any case, the importance of structural biology for science is clear from the large number of Nobel prizes awarded to the field over the years.
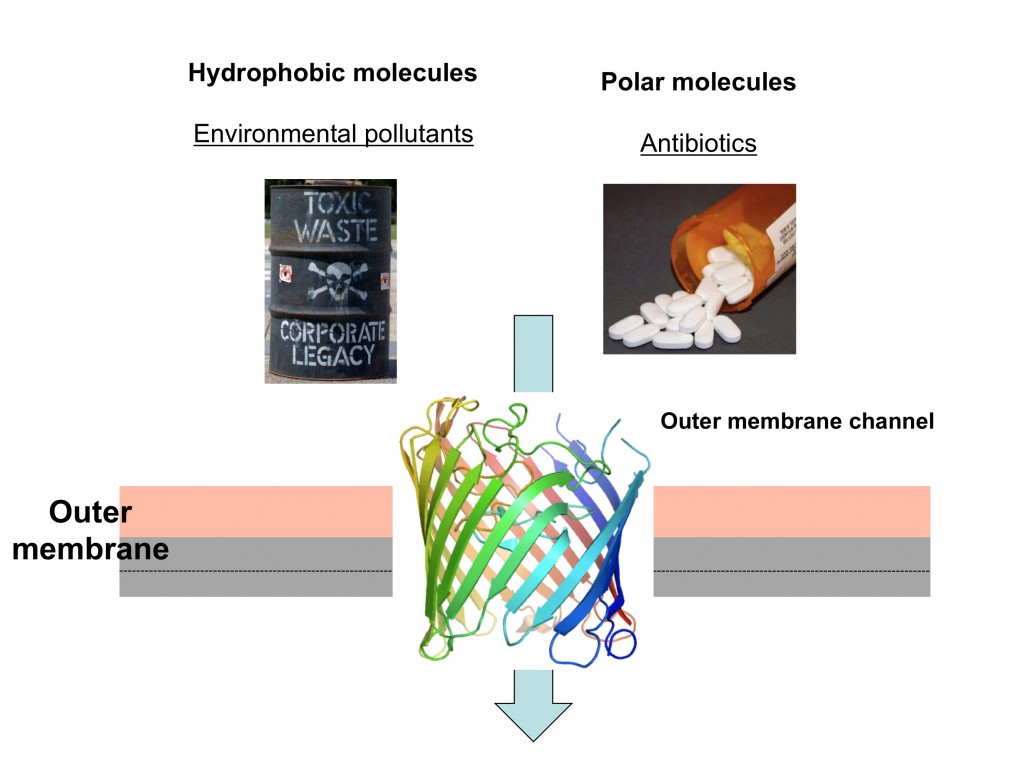
What do the cleanup of oil spills and the treatment of many bacterial infections have in common? The answer is that both processes depend on the efficient passage of bacterial membranes by small molecules.
Gram-negative bacteria are surrounded by two lipid membranes, which are termed plasma membrane and outer membrane. The outer membrane borders the cell and is a very efficient and sturdy barrier that protects the cell from noxious substances in the external environment, such as bile acids in the case of E. coli bacteria living in the gut. However, since bacteria also require nutrients for growth and function, protein channels are present in the outer membrane to allow the uptake of such small molecules. In our work we use X-ray crystallography to determine the atomic 3D structures of the channels, most of which are shaped like hollow barrels. Based on the structures we propose transport models, which we then test by characterisation of mutant proteins.
Many Gram-negative bacteria are able to use industrial pollutants such as oil as food sources, a process called biodegradation. The enzymes that catalyse these remarkable processes are located inside the cell but not much is known about how the pollutants enter the cell in the first place, something that is clearly required before they can be degraded. We study the highly specialised channels that mediate the uptake of these water-insoluble (“hydrophobic”) molecules. In addition, we are interested in discovering cellular adaptations that allow biodegrading bacteria to grow on these toxic compounds. We think that this research may lead to insights that will aid the design of bacterial strains that are optimised not only for bioremediation but also for important other processes such as production of biofuels.
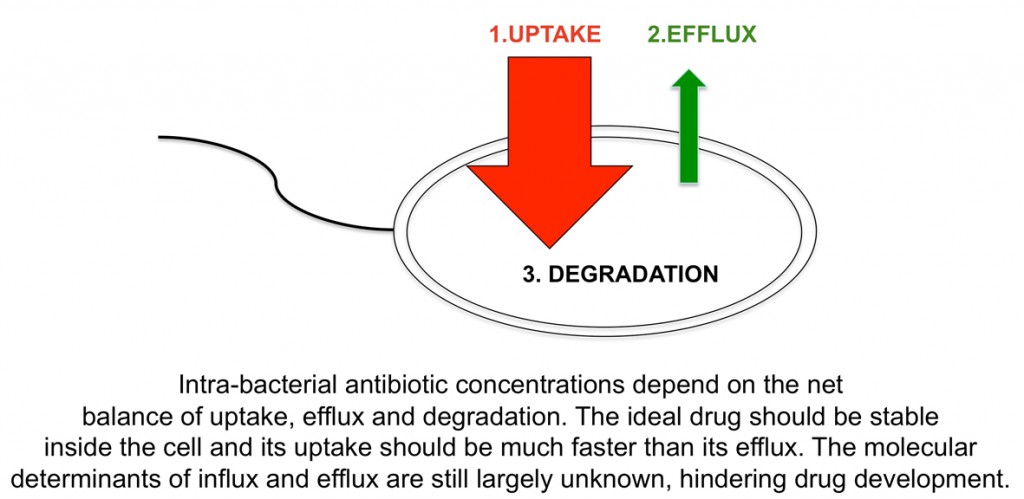
The other main focus of research in my lab is to understand how antibiotics “hijack” outer membrane channels to enter bacteria. Being water-soluble, antibiotics are dependent on protein channels for membrane passage. Bacteria that are under antibiotic pressure will often change or remove the channels through which antibiotics pass, resulting in resistance.
Movie showing ampicillin movement through E coli OmpF protein channel. The view is from the outside of the cell. Movie made by Matteo Ceccarelli (University of Cagliari).
In concert with other mechanisms such as enzymatic degradation and increased efflux by pumps, this acquired antibiotic resistance has the potential to become a huge and global problem in public health. New drugs are therefore urgently needed. The problem is that not nearly enough new drugs are currently in pharmaceutical pipelines, due to the costly and risky nature of antibiotic development. However, pharmaceutical companies are starting to realise that the fundamentals of drug design need to change, and that they have to collaborate with academic labs that are studying the basic biology of small molecule membrane transport.
My lab is participating in an exciting, EU-funded joint venture between big pharma, small biotech firms and academic labs aiming to understand the influx/efflux of drugs in a number of pathogenic Gram-negative bacteria. Beyond the potential benefits for drug design, it is hoped that this project will change the way in which industry and academia work together to benefit public health.
Links
Royal Society Wolfson Merit Awards: http://royalsociety.org/news/2013/new-wolfson-research-merit-awards/
Bert’s ICaMB homepage: http://www.ncl.ac.uk/camb/staff/profile/bert.van-den-berg
Newcastle Structural Biology website: http://sbl.ncl.ac.uk/people/bert_research.shtml
Structural Biologist Nobel Prize Winners: http://www.ebi.ac.uk/pdbe/docs/nobel/nobels.html
IMI TRANSLOCATION project: http://www.imi.europa.eu/content/translocation