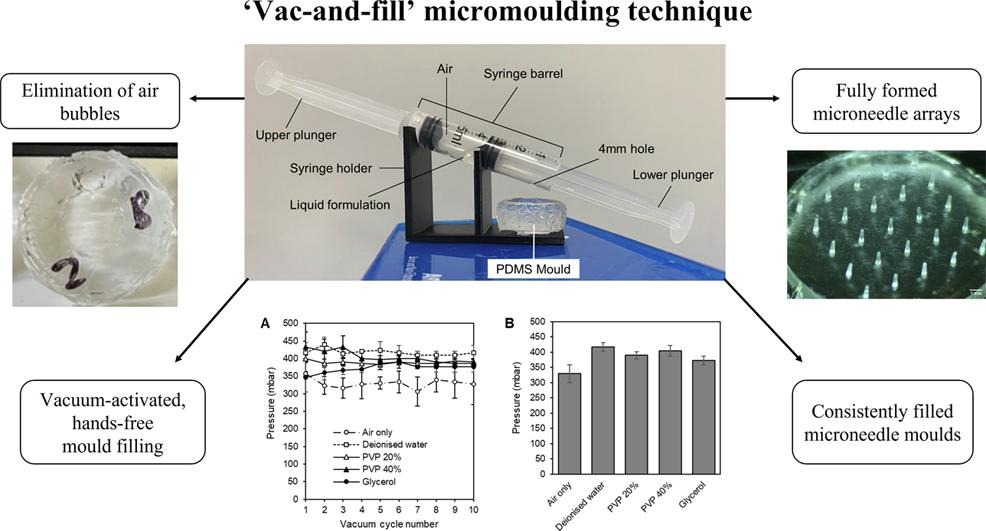
Abdelghany TM, Vo N, Vukajlovic D, Smith E, Wong JZ, Jackson E, Hilkens CMU, Lau WM, Ng KW, Novakovic K. Engineering and in vitro evaluation of semi-dissolving, hydrogel-forming polymeric microneedles for sustained-release drug delivery. Int J Pharm. 2025:125932. https://doi.org/10.1016/j.ijpharm.2025.125932

In our latest paper, we describe a microneedle formulation that utilises two polymeric domains: a soluble one and an insoluble one. The insoluble domain is chemically crosslinked and traps the soluble polymer, along with the drug, within it. This combination creates a microneedle array patch that can release a drug for over 2 months.
It can contain a significantly larger dose than microneedles where the drug is contained within the microneedle tips only (e.g., detachable microneedles). The drug reservoir in the backplate makes this possible to support extended release. It uses one-pot synthesis, a mild hydroalcoholic solvent system and mild temperatures to aid manufacturability and drug stability.
For the first time, we were able to see, on video, how the microneedles released the drug and swell as they hydrated. These videos are buried in the supplementary files for the paper, but I thought it worthy of sharing more widely here:
Videos from Abdelghany et al. (2025). Reused under a Creative Commons licence.
We would like to thanks everyone who’s contributed to this paper. Big thanks to the EPSRC and Innovate UK for funding this work.