By: Caitlin Allison
LabCorp Drug Development
Pharmaceuticals are all around us; we probably all have taken a drug, received a vaccine, or known someone who has. These small molecules can not only improve the quality of life but also save lives. All these drugs require drug developmental processes.
LabCorp Drug Development, formerly Covance, is a Contract Research Organization (CRO). It delivers a range of experimental packages for nonclinical, preclinical, and clinical services to many pharmaceutical and biotechnology companies.
I was located at Harrogate North Yorkshire, a site which specialises in preclinical pharmaceutical development. At this site, I was a Dose Analyst- an employee who investigated formulations for preclinical studies. I analysed formulations via High-Performance Liquid Chromatography (HPLC).

Application Process
I first saw LabCorp advertising many placement opportunities on Indeed in October. My initial application required a CV and a brief covering letter; with help from CV workshops run by Kings Gate I submitted my application and waited for a response.
My first phone call was with the university recruitment specialist at LabCorp Harrogate. This call was to confirm my job application, outline the job role, and understand the preclinical testing stages.
I later received a link to complete a pre-recorded video interview. This could be conducted at any time within a timeframe. It consisted of 5 general competency-based questions, in which you could record a video and upload it in the comfort of your own home!
Afterward, I was short-listed for an interview over Zoom and met my manager and supervisor. The questions were somewhat competency-based, but included technical-based questions;
- Conversion of grams into micrograms
- Concentration calculations
- My understanding of GLP
- explanation of chromatography
- Precision vs accuracy and appropriate statistical tests
I was offered the internship just before Christmas and gleefully, I accepted.
Job Role
A dose analyst is responsible for testing the homogeneity, stability and achieved concentration of formulations required for preclinical trials. HPLC is a favoured method of analysis as it is very selective in nature due to subtle changes in gradient programs, different mobile phases, and a wide variety of column chemistry being available. In some instances, it’s even possible to see degradation peaks, which is interesting to see in a stability study.

Photo by asikkk on Unsplash.
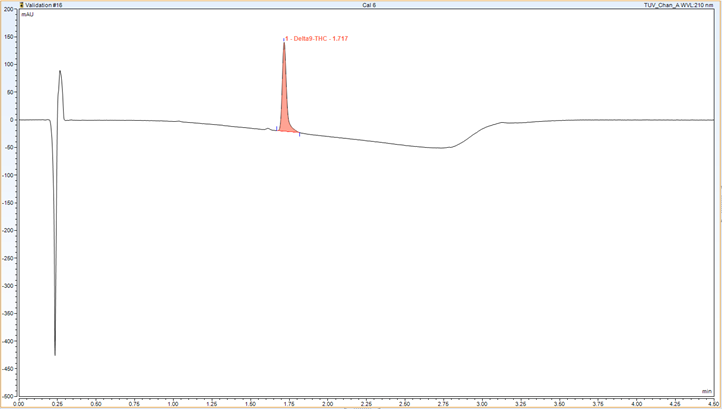
I completed a project for the university based on Δ9– Tetrahydrocannabinol (Δ9– THC), an established controlled drug. This defeated any issue regarding client confidentiality of novel pharmaceuticals.
Δ9– THC is an addictive drug and therefore can be used as a positive control comparison for addiction studies in pharmacology. It was my duty to ensure Δ9– THC could be formulated to the correct concentration and be homogenous for preclinical pharmacology studies.
My project required me to create a method of analysis via HPLC, selecting appropriate analytical methods as well as optimum conditions on a HPLC instrument. I tested the stability of this formulation to see if there was a way in which the formulation could be stored for a longer period making formulation and dosing, and subsequent analysis works efficiently.
Highlights
LabCorp offered workshops for employees on the global intern program which promoted ways of time management and prioritising workloads. These workshops also showcased ways to communicate effectively and professionally; I often found myself communicating regularly with clients (pharmaceutical companies) and our own study director. Some of the formulations I analysed came from different LabCorp sites including Madison in USA and Münster in Germany. My communication had to be clear and legible.
I managed to network with people in other departments e.g., metabolism, and managed to talk to the intern from the clinical testing site in Leeds. Networking offered me a great opportunity to see what other jobs are out there. The drug development industry is much vaster than I initially thought!
Difficulties
One of the things I had to quickly accept was in science sometimes your results aren’t even in the specification. At first, I took this personally; Was I bad at my job?
However, as part of GLP, if I experienced out-of-specification results, a co-worker investigated my data capture, the test item, a laboratory inspection, and instrumentation. There was a lot that could potentially go wrong. Sometimes no analytical error could be found. My heart would be relieved as these results were out of my control.
However, out-of-specification results were at times found to be because of my error. This was obviously disappointing to hear but was also a potential learning curve I could experience. Quality issues could be raised easily and weren’t catastrophic. I slowly learnt to accept this was a part of the job role.
Developed Skills
Working full-time let me see the routine setbacks you face in science. This helped me develop appropriate ways of responding to problems and not immediately thinking catastrophically. Out-of-specification results had to be reported timely and accurately, stating details very thoroughly. This has helped me work very efficiently paying great attention to detail, something which will be beneficial in the future.
I calibrated equipment and recorded data quantifying the statistical significance of precision and accuracy, this coincided with GLP requirements. This let me appreciate the percentage error in scientific equipment and optimized my laboratory performance e.g. correctly holding a pipette.
I was doing laboratory experimentation on a daily basis; I became very dextrous and learnt to work very efficiently. If I noticed errors, I could offer a scientific explanation of observations seen, and in some cases, I could troubleshoot a HPLC independently! Something I couldn’t dream of doing this time last year!
Future Aspirations
I really appreciated working for LabCorp Drug Development, they gave me a fantastic learning opportunity and I saw how big the drug development industry really is. I have an appreciation for the work I did after seeing the therapeutic areas the pharmaceuticals I was working on treated, these drugs would not only improve the quality of life for a lot of people but would also save lives. I think there are a lot of job possibilities in this sector I would like to explore, and the dose analysis intern was a good introduction and allowed me to see how these jobs interlinked. I believe I developed personally and professionally. I know I want to work in the industry again.
Interested in following in Caitlin’s footsteps? You can do a placement year as part of all of our undergraduate degrees. Caitlin is a BSc Biochemistry student with Professional Placement Year.

