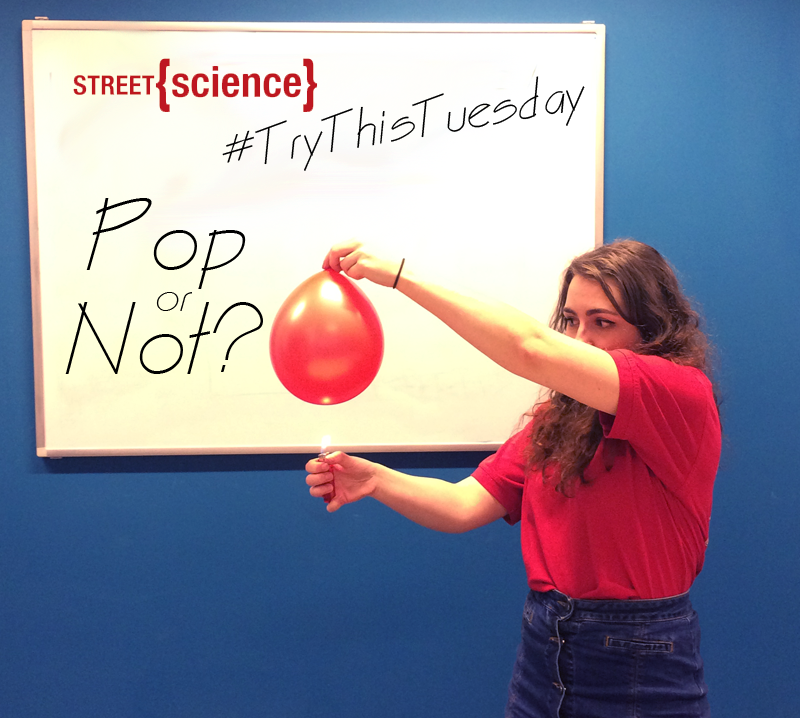
Today we will be experimenting to see what happens when you put a lighter or a flame underneath a balloon filled with two different states of matter: air and water.
You will need two balloons, some water and a lighter
- Blow up one of the balloons with air and tie it up.
- Fill the other balloon with a little bit of water, blow it up the rest of the way and tie it up.
- Hold the lighter under the balloon with the air in it and see what happens. Be careful as it should pop!

Balloon with air in - Light the lighter under the balloon with some water in it, be careful to hold the lighter under the part of the balloon where the water is. The balloon won’t pop!

Balloon with water in
The Science
This happens because water can absorb heat a lot easier than air and is a better conductor of heat. Water keeps the heat away from the balloon. This is called its ‘heat capacity’ and is why water is often used to cool things down in places such as power plants. The air is not very good at absorbing the heat, so the balloon heats up and pops!