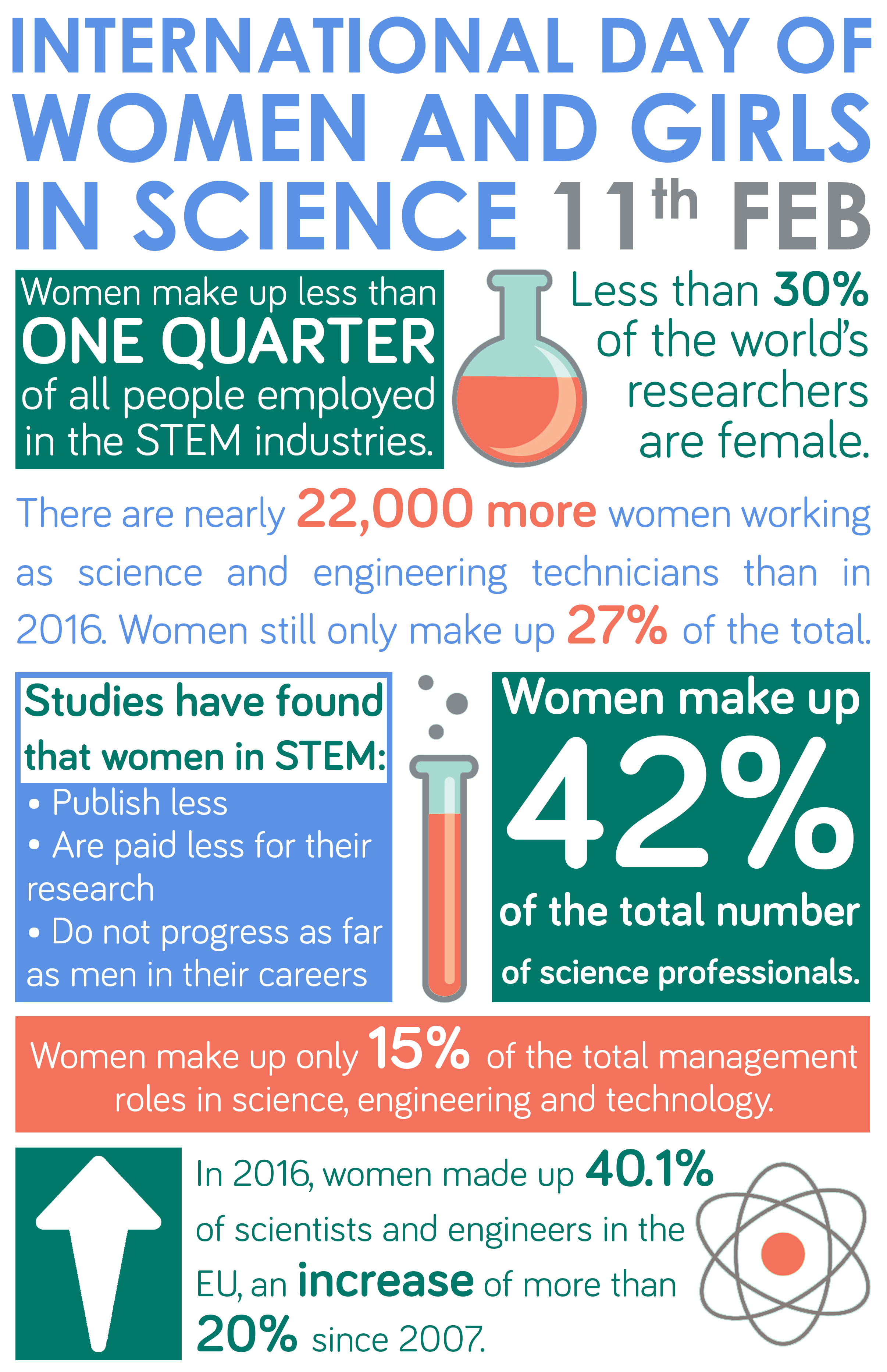
Organising your time on a course with high contact hours, such as engineering, can seem daunting. Farah Nabilah gives us an insight into how she manages her time as a Stage 2 Civil Engineering student.
I am currently studying BEng Civil Engineering and something I often get asked about is how I find the work/life balance. Initially, I found University life to be very different to school in a sense that you must be independent. At school, the teacher would go through the material and after, we would go through the questions in class. At university, the lecturer will go through the material and a few examples in lectures, but you are expected to go through the practice question in your own time. We have the opportunity to ask the lecturers any questions during tutorial sessions. The tutorial session is more of an informal session where you can ask the lecturers or any PhD students one to one questions. After a couple of weeks of being at University, I started getting used to this system.
 Stage 2 is different to stage 1 as there are less lectures to attend but more work to be done outside; in particular, group work. You also have more design modules as opposed to just learning mostly theory like in stage 1. For me, I did spend a lot more time studying outside of class to try and understand these concepts, so I do have to organise my time well. I usually volunteer on Sunday to give myself a break from doing University work.
Stage 2 is different to stage 1 as there are less lectures to attend but more work to be done outside; in particular, group work. You also have more design modules as opposed to just learning mostly theory like in stage 1. For me, I did spend a lot more time studying outside of class to try and understand these concepts, so I do have to organise my time well. I usually volunteer on Sunday to give myself a break from doing University work.
To switch off I like to take walks and get some fresh air. The University is close to Leazes Park and the city centre which is great as I can take a walk there with my friends to refresh after a session of studying. I also like to watch TV shows when I have time. I work as a student ambassador and I help out during events such as open days and post application visit days. I really enjoy doing this as it helps me to develop my communication skills and I get to share my experience at University with other people.
I think that in order to be able to manage your time whilst still having fun, it is important to stay organised. I keep a planner, so I know what events I have on each day and make sure that I don’t miss out anything important. Also, I try to prioritise tasks; I do tasks that are more important first. Most importantly, I think it’s beneficial to do the work as soon as possible so you are not left with a large amount of workload and don’t end up being stressed.
Find out more about Newcastle University’s Civil Engineering degrees here.


 The Water
The Water
 The milk
The milk
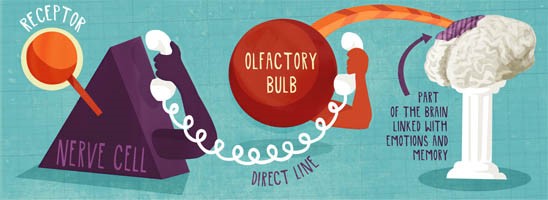


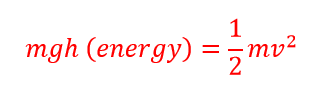
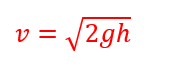
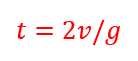 So when you are flipping your pancakes today think of all the wonderful physics behind that perfect flip!
So when you are flipping your pancakes today think of all the wonderful physics behind that perfect flip!