Recently myself and my fellow STEM Graduate Ambassadors, Ellie and Will, were lucky enough travel to Hong Kong to take part in the British Council organised Science Alive Festival at the HK Science Museum. As the festival’s theme this year was This Pale Blue Dot – we also took two Earth Science students, Hannah and Elizabeth.

To take full advantage of this wonderful opportunity we also reached out to some schools with the help of Anthea, Newcastle’s Hong Kong Recruitment Manager. With 20 STEM workshops booked in, we took to the skies with our bags packed with as much kit as we could carry including a drill, over 20 metres of rope and a dinosaur costume.

During the first three days, our time was spread amongst seven schools. We were delighted to see how bright and engaged the children were throughout our workshops, particularly in the primary schools. Each school was extremely welcoming, many of them gave us banners with their school logo on and other gifts. The Vice Principal of one school even took us to a nearby bakery after the visit to treat us to a local delicacy – a warm egg tart.

The weekend snuck up on us and before we knew it we were performing our science show: The Story of Earth, at the Science Alive festival. I was setting Will on fire (don’t worry, it didn’t hurt him although I did actually singe his arm hair at one point) and Ellie was freezing flowers with liquid nitrogen. Meanwhile Hannah and Elizabeth were science busking; entertaining and educating children with science demonstrations.

We also ran training workshops for teachers. We taught primary school teachers how to think outside the box to raise aspirations and teach science in a more interactive and fun way. At the Hong Kong University of Science and Technology, we trained the students on good science communication practices, from science busking to written texts. We explained how they could simplify their language to make their research more accessible and easy to understand.

During our ten days in Hong Kong we had an absolute blast, educating thousands of children and adults and learning all about their culture and styles of teaching. From bursting a 3-foot confetti-filled balloon to teaching children how to make honeycomb, from visiting the highest bar in the world to seeing 10,000 Buddha statues in one room, it was an unforgettable experience.

We feel honoured to have been given the opportunity to share our activities with another part of the world and hope next year’s team will be able to have a similar adventure.



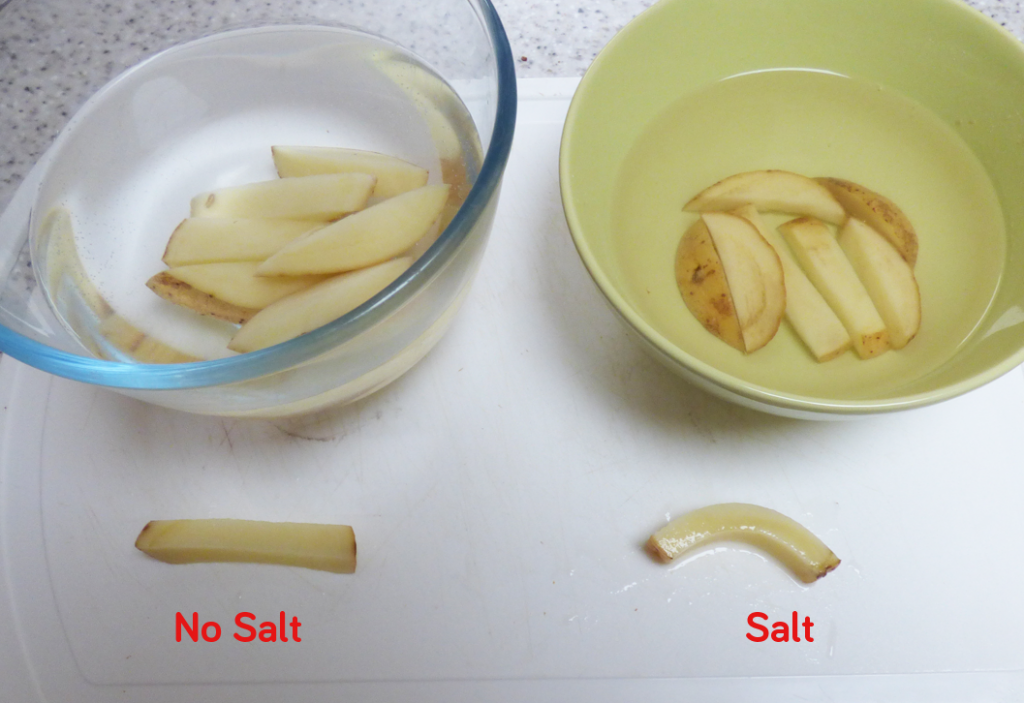
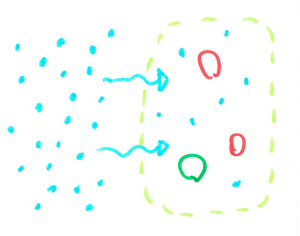
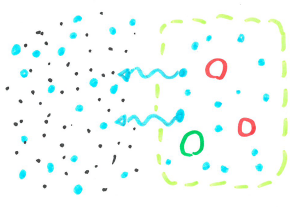
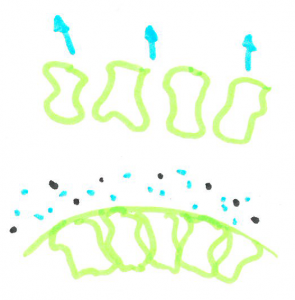 When cells are filled with water they are quite rigid and packed closely together making a fairly sturdy chip. When the cells are dehydrated, they are smaller leaving space between cells, allowing the chip to bend without snapping.
When cells are filled with water they are quite rigid and packed closely together making a fairly sturdy chip. When the cells are dehydrated, they are smaller leaving space between cells, allowing the chip to bend without snapping.



 Try making different flavours of ice cream by swapping the vanilla extract for strawberry or mint extract or even cocoa powder for chocolate ice cream. You could also try adding chocolate chips.
Try making different flavours of ice cream by swapping the vanilla extract for strawberry or mint extract or even cocoa powder for chocolate ice cream. You could also try adding chocolate chips.