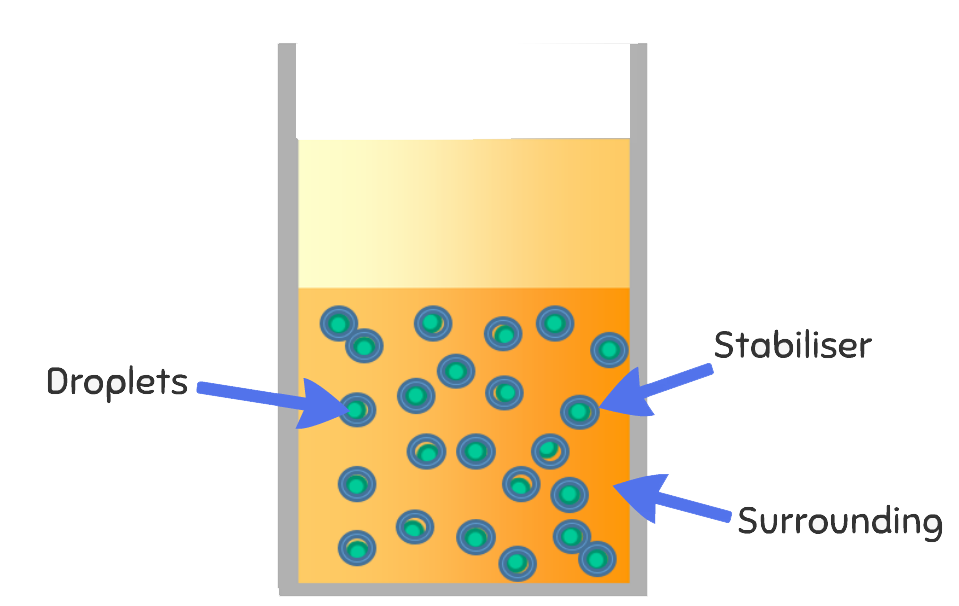
Second year Marine Technology student Yanis talks us through what a typical day is like for him studying and living in Newcastle.
 Hey guys,
Hey guys,
I’m Yanislav, but most people call me Yan or Yanis. I am currently a second year student and I’m studying a masters programme in Marine Technology w/Naval Architecture.
Newcastle was my first choice university for many reasons, the biggest one being the feeling of community and connection between the course and student. It really does feel like your degree doesn’t just matter to you, which makes studying much more enjoyable!
Another benefit of being here is the incredible city itself. From stunning architecture and high culture art installations/indie digs, to the wide array of bars/social spaces, restaurants and shops, it’s easy to find something new to do or a place to relax.
University and student life are, as everyone likes to joke, very different to anything you will have come across before.
Most of my days will start anywhere between 9am and 12pm and typically I will have at least 3 hours of timetabled activity, but this can easily be as much as 7 hours at the busiest times of the semester. The amount of contact time may seem big, but this is a blessing in disguise really! On any given day I will tend to do 2-3 hours of additional work in the many libraries/study areas around the university and I use this to keep on top of the lecture content and complete any additional work needed to do well in assignments and exams.
The lectures and content studied are quite varied; compare pure Engineering Maths with a wordier Production Management module; which provides a nice mix of study. Personally, I really enjoyed the Marne Engineering and Naval Architecture modules, as they had a good balance between the science and maths you’d expect to study.
Something I didn’t expect to do was coding and some of the Computer Aided Design, like modelling a single-cylinder engine. Although not easy to start with since I had no prior experience, I did find both very interesting and satisfying once I got the hang of it.
Outside of academia I am a member of the Defence Technical Undergraduate Scheme (DTUS), as a Royal Navy sponsored student. This means I attend the local naval base once a week and develop the skills I will need in order to be a technical officer. Alongside this I also get the opportunity to lead and develop various Adventurous Training, specifically for me Offshore Sailing.
In my downtime I like to experiment with cooking, picking up various bits and pieces to try from the massive Grainger Market. I also enjoy watching the rugby at the nearby Newcastle Falcons Kingston Park stadium and there is also the compulsory night “Ooot in Toon” which you will no doubt experience at least once (a week).
Feel free to send me a message on Unibuddy, you are more than welcome to ask me more about my daily happenings, the course or any concerns you have about the step up to university!
Cheers for reading!
Yanis











 Hey guys,
Hey guys,











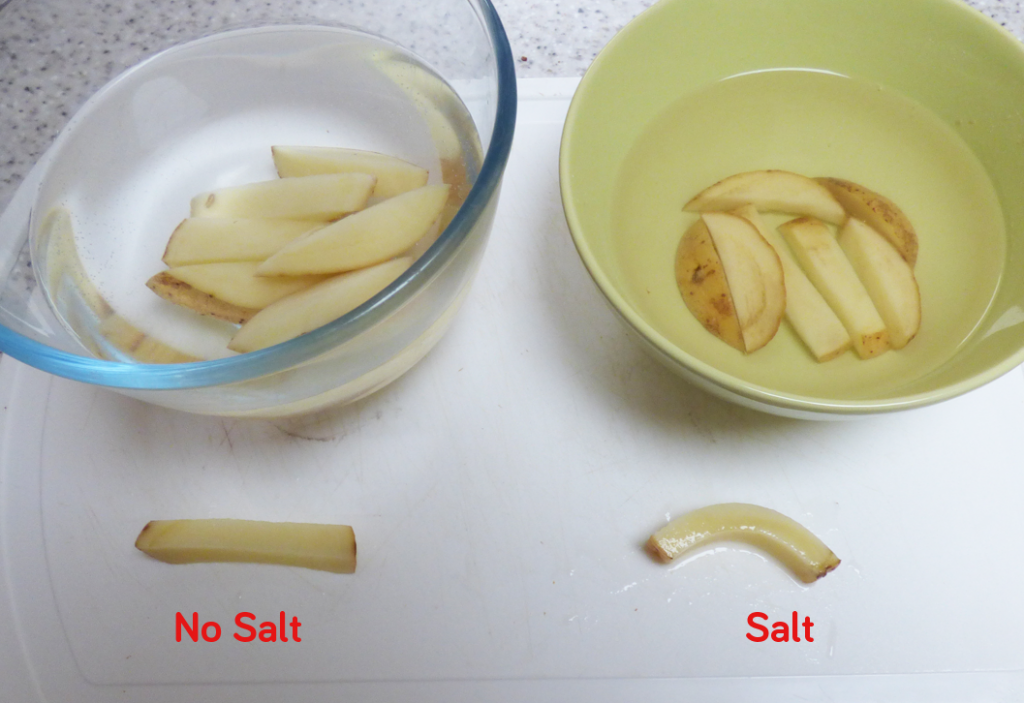
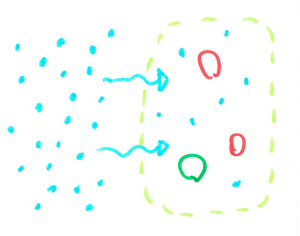
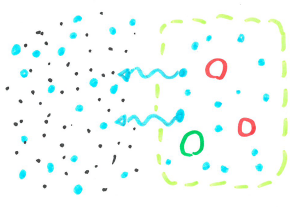
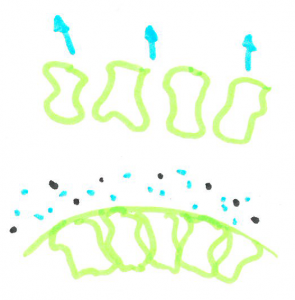 When cells are filled with water they are quite rigid and packed closely together making a fairly sturdy chip. When the cells are dehydrated, they are smaller leaving space between cells, allowing the chip to bend without snapping.
When cells are filled with water they are quite rigid and packed closely together making a fairly sturdy chip. When the cells are dehydrated, they are smaller leaving space between cells, allowing the chip to bend without snapping.

 Try making different flavours of ice cream by swapping the vanilla extract for strawberry or mint extract or even cocoa powder for chocolate ice cream. You could also try adding chocolate chips.
Try making different flavours of ice cream by swapping the vanilla extract for strawberry or mint extract or even cocoa powder for chocolate ice cream. You could also try adding chocolate chips.