Our Try This Tuesday series of experiments to try at home is back with a colourful water-based experiment from Street Scientist, Ailie.

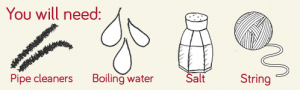
You will need:
- Red, yellow and blue food colouring
- Kitchen roll
- 6 Clear cups, roughly the same size
- Add water to three of the cups so they are 3/4 full. Then add 5 drops of red food colouring to one cup of water, blue into the second and yellow into the third.
- Place the cups in a circle with the empty cups in-between the ones with water in (You may want to put some paper underneath the cups if you are worried about spills).
3. Take 6 sheets of kitchen roll and fold them twice to make a thick strip as below. You may want to cut them to be a bit shorter if you are using small cups, I cut off the bottom 1/5th.

4. Place one end of each strip of kitchen roll into one of the cups with water in and the other end into an empty cup next to it. Can you predict what colours might form in the empty cups?
5. Wait an hour then check back in to see if your experiment is working! Mine looked like this:

Eventually the empty cups will be as full as the cups feeding into them!

Colour Theory
Did you notice something the 3 colours you started with had in common? They are the primary colours. Therefore, mixing them creates the 3 secondary colours – purple, green and orange.
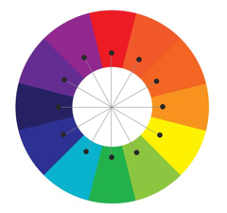
This gives us a simplified version of a colour wheel. The colours opposite each other on the wheel, and in our cup circle, are ‘complimentary’ meaning they contrast one another i.e. purple and yellow.
If we were to add more empty glasses in between the colours we have here we would make tertiary colours!
The Science
The water moves up the paper towel through a process called capillary action which is the ability of water to flow through narrow spaces, even against gravity! Capillary action works as molecules in liquid like to stick together (cohesion) and also like to stick to walls of a tube (adhesion). Together these forces act to propel the liquid through the tube or narrow space. The narrower the space, the quicker the water moves and the higher up it can go.

Plants rely on capillary action to move water all the way up from their roots to the leaves at the very top, where it is needed for photosynthesis (the production of glucose for energy). Just as humans have blood vessels to carry important substances around our body in the blood, plants have a tissue called xylem which is made up of millions of tiny tubes. Water moves up through the tiny cubes by capillary action, without wasting any of the plant’s energy.

One way to easily see capillary action working in the xylem is to cut the very bottom off the stem of celery or cabbage and put it into some water with food colouring. Given enough time, you will see the coloured water move to the top of the plant and stain the leaves. When a plant has been picked it is no longer undergoing photosynthesis and producing energy, therefore we have shown that the water is moving up to the top of the plant by a passive process.
Kitchen roll is designed to be very absorbent meaning it is able to hold lots of liquid – great for kitchen spills. To be able to do this, there are lots of little spaces in-between the fibres in kitchen roll which fill with water. Together they form the narrow spaces which the water uses to move up the tissue and into the adjacent empty cup. There, the colour mixes with the water from the cup on the other side to form our secondary colours.







 The Water
The Water
 The milk
The milk