To celebrate International Talk Like a Pirate Day we have put together a virtual treasure hunt that you can do from a phone or computer.
For this treasure hunt you will need to solve clues to find co-ordinates to the next stop . The first three people to make it to the end of the hunt will win the treasure of a Street Science Busking Kit which is filled with equipment to make amazing science demonstrations at home!
Using Co-ordinates
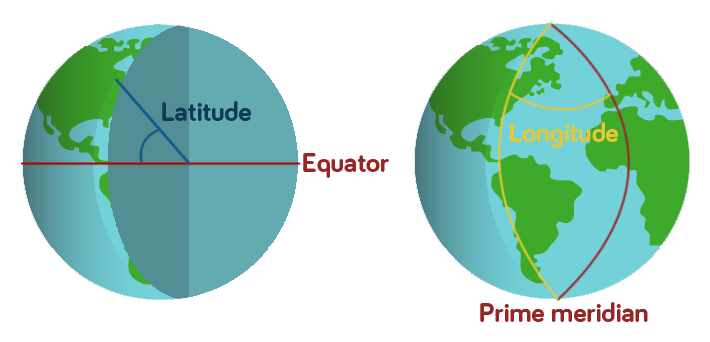
Geographic co-ordinates allow every place on Earth to be identified with a set of numbers. The system that we’re using for this treasure hunt gives every point a latitude number and a longitude number.
If you were to draw a line from your point on the globe into the centre of the Earth and another from the centre of the Earth to the Equator, the angle between these two lines gives you the Latitude.
Longitude is measured slightly differently. There is an invisible line running from the North Pole to the South Pole through the Royal Observatory in Greenwich, London, known as the Prime Meridian. All points along this line have the longitude 0. The longitude of other points are calculated as the angle east or west from the Prime Meridian.
So Newcastle Upon Tyne, where we’re starting our treasure hunt from, has the co-ordinates 54.97, -1.61 because it is 54.97 degrees from the Equator and 1.61 degrees to the west of the Prime Meridian. Going west of the Prime Meridian or south of the Equator gives the co-ordinate a negative value.
The Treasure Hunt
Try to solve the clues to find the co-ordinates for the next place. Once you’ve got co-ordinates type them into Google maps (just like this: 54.97, -1.61 with latitude first) and make a note of where you’ve got to.
This will test your research skills as well as your maths skills, feel free to use Wikipedia and a calculator.
Destination One
The latitude for the first place is 57.14 divided by the number of universities in Newcastle Upon Tyne.
The longitude is equal to 10 – 90.65.
Where are you?
Destination Two
To find the latitude of our second destination, take the last two digits of the year the first man walked on the moon, half this number then add 3.32
The longitude can be found by taking the year Newcastle University became independent from Durham University away from the year that the oldest part of the university (the School of Medicine and Surgery) was established and adding 6.52.
What amazing feat of engineering are you near?
Destination Three
For the latitude of our next place, take away 3.1 from the distance that Destination Two stretches in miles.
The longitude can calculated by the number of different countries that students at Newcastle University have come from divided by -2.
Which wondrous forest have we arrived at?
Destination Four
The latitude of this place can be uncovered by multiplying the number of countries that share Destination Three by 5 then adding 1.234
The longitude is equal to the number of reptile species that have been discovered in our previous destination divided by 63 then add 0.053
What amazing discoveries were made here?
Destination Five
The above place is often known by an abbreviation of four letters. If you convert these letters to numbers (eg. a=1, b=2, c=3 etc.) and square the second number, then add 0.197 you will have the latitude of our next destination.
To find the longitude, convert the first letter of the abbreviation to a number and take this away from 58.274.
Which incredible man made structure are you looking at now?
Destination Six
For the latitude of our final place – where the treasure is buried – you will need to divide the total height of the structure we just saw (in metres) by -46.1.
To find the longitude, simply take away 16 from the number of floors in this structure.
Where is our treasure buried?
If you think you’ve cracked it, either send us an email to stem@ncl.ac.uk or comment below with the six places the co-ordinates led you to!