March 22nd marked a grim first anniversary: what we now know is the deadliest Ebola outbreak to date was officially announced by the WHO in Guinea. Since then, news reports have featured the dramatic and worrying outbreak of Ebola virus infections in West Africa. In our blog post this week, ICaMB’s Dr Anjam Khan reviews this deadly but scientifically fascinating killer and reflects on his recent experiences discussing the virus in the media.
March 22nd marked a grim first anniversary: what we now know is the deadliest Ebola outbreak to date was officially announced by the WHO in Guinea. Since then, news reports have featured the dramatic and worrying outbreak of Ebola virus infections in West Africa. In our blog post this week, ICaMB’s Dr Anjam Khan reviews this deadly but scientifically fascinating killer and reflects on his recent experiences discussing the virus in the media.
By Dr Anjam Khan
Prophecies of Science Fiction
 As a school kid I was fascinated by science fiction and the visionary predictions made about future technology by the fathers of this genre, Jules Verne and H. G. Wells. Science fiction writers have also forewarned of global catastrophes ranging from those caused by climate change, to the annihilation of the human race by a virus concocted in the lab of a mad scientist! A book called “The Andromeda Strain”, by the best-selling author Michael Crichton, captivated me. The story was set in Arizona. It followed a team of scientists in a secret high containment lab trying to control the apocalyptic spread of a deadly extra-terrestrial microbe. The infection rapidly caused lethal blood clotting in its victims, or “disseminated intravascular coagulation” to us nerds! The crystalline alien microbe “mutated” at an extraordinarily high frequency, almost instantaneously acquiring new biochemical skills such as the ability to digest through plastics and rubber seals. These are attributes synthetic biologists dream of constructing today in “environmentally friendly” bugs to degrade plastics!
As a school kid I was fascinated by science fiction and the visionary predictions made about future technology by the fathers of this genre, Jules Verne and H. G. Wells. Science fiction writers have also forewarned of global catastrophes ranging from those caused by climate change, to the annihilation of the human race by a virus concocted in the lab of a mad scientist! A book called “The Andromeda Strain”, by the best-selling author Michael Crichton, captivated me. The story was set in Arizona. It followed a team of scientists in a secret high containment lab trying to control the apocalyptic spread of a deadly extra-terrestrial microbe. The infection rapidly caused lethal blood clotting in its victims, or “disseminated intravascular coagulation” to us nerds! The crystalline alien microbe “mutated” at an extraordinarily high frequency, almost instantaneously acquiring new biochemical skills such as the ability to digest through plastics and rubber seals. These are attributes synthetic biologists dream of constructing today in “environmentally friendly” bugs to degrade plastics!
The Devastating Realities of Ebola The deadly Ebola virus outbreak is not from the realms of science fiction but written by nature. This outbreak has shocked the world not only because of the high mortality rates, but due to the horrific disease it causes. The virus has quickly spread and brought the three West African countries, Guinea, Sierra Leone, and Liberia, to the their knees, and to the brink of economic collapse.
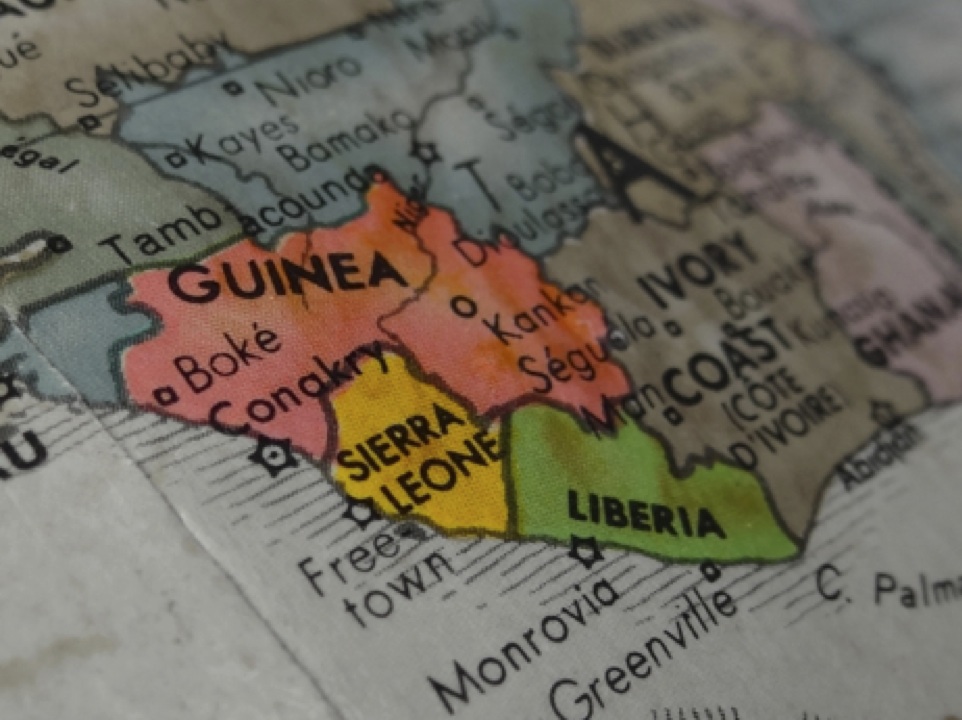
Map of West Africa with Guinea, Sierra Leone, and Liberia highlighted. Image from copyright free – morguefile.com
Reports of the first cases of Ebola in Europe and the US brought panic and fear to those who previously thought this deadly plague was confined only to West Africa. Undeniably, governments and healthcare organizations were all taken by surprise, and were ill prepared to rapidly deal with the disease or indeed its global threat. Why had Ebola taken the world by surprise? The warning signs have been there for almost four decades! The first case of the disease was described in 1976 in Zaire (now the Democratic Republic of Congo), near the Ebola River from where the virus gets its name. With the present outbreak there have been over 23,000 reported cases with approximately 10,000 deaths. The human toll in fatalities has been almost 10 fold greater in the current outbreak than all the previous outbreaks put together!
Politics of Disease Control and Media Engagement The world hesitated in responding to the emerging Ebola crisis, naively hoping the disease would burn itself out. This did not happen and the number of newly infected individuals increased week on week. Some politicians and members of the media fuelled the fears of the masses. Our government hurriedly announced it was establishing four NHS hospitals, including the Newcastle Royal Victoria Infirmary, as Ebola centres with highly trained staff and fully equipped facilities to deal with Ebola cases. Furthermore, compulsory thermal scanning of travelers arriving in airports from selected destinations was introduced, in a bid to spot anyone with a fever and potentially infected with Ebola. There are, however, many other infections and health conditions which can also induce severe fever.

Thermal cameras to detect passengers with high fevers were recommended in airports for screening for potential Ebola infected individuals. Photo Credit: cdc.gov
Hence, in reality this screening approach was only of limited value in identifying Ebola infected travellers, and perhaps served more as a placebo to allay the fears of the public. For example, Thomas Eric Duncan was thermally screened upon arrival in Texas, and he later developed full-blown Ebola and sadly died of the disease in Dallas. Two nurses treating him also became infected.
The national and local news media wanted to find out more about Ebola and the control measures being put in place. With a background in infectious diseases, I was asked to provide insight into the virus and the disease it causes. Normally I would run a mile from being in the public eye. But given some gentle encouragement, decided on this occasion to raise my head above the comfort zone of the academic parapet, and try to provide sensible and factually correct advice on the virus and the disease. Following my first interview with a local newspaper the requests for interviews increased, and within a few weeks I had given three television, four newspaper articles (Northern Eco, Chronicle, The Week), and a radio interview! This included one live TV interview for BBC News 24 via a satellite link, which was petrifying! It was challenging not only because it was being transmitted live, but due to the technical arrangement – but that’s a blog for another day! Speaking to the media was a brand new experience for me. It was certainly a steep learning curve and I realised the importance of “sound-bites”: you can be interviewed for 20 minutes but the editor may only select two 15 to 20 second excerpts for airing. Furthermore you quickly realise you have no editorial control of the content or indeed the context of your quotes!
Pirates of the Immune System
The first Ebola outbreak in 1976 was identified in Central Africa, and it has now spread to Western Africa. This distance at first glance may not sound very far, but to put this into perspective, it is comparable to the distance between Newcastle (UK) and Quebec (Canada) or between Newcastle and Cairo (Egypt)! A key factor contributing to this spread could be the fruit bats, which harbor the virus, flying and migrating to new habitats. For reasons unknown as yet, some fortunate individuals can recover supported by simple hydration therapy. These survivors provide a hugely invaluable resource of biological information, and will undoubtedly provide important insights into understanding the 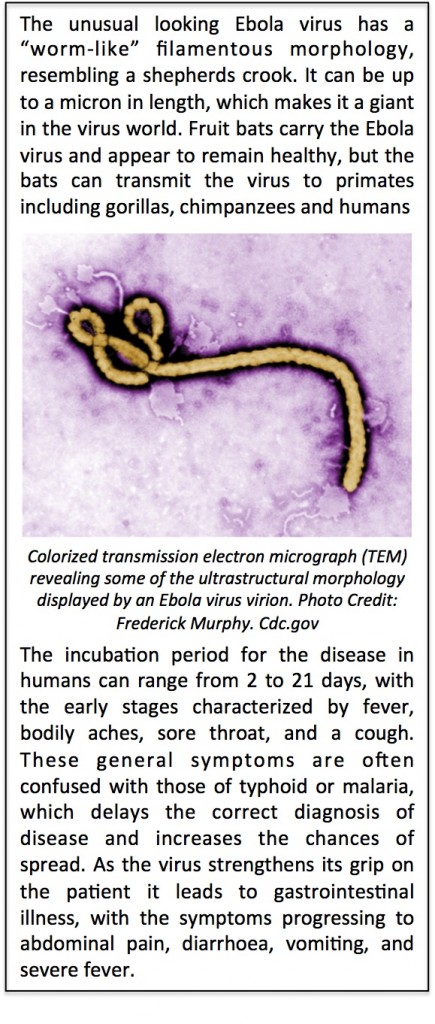 immunological correlates of protection or the genetic basis of resistance to Ebola. But sadly for the vast majority of infected individuals, the disease is devastating. The cunning virus “pirates” the immune system and incapacitates the anti-viral machinery of the immune cells by blocking the “interferon alarm” from sounding in target cells. The virus then triggers a “cytokine storm” triggering the release of potent inflammatory molecules into the circulatory system that wreak havoc throughout the body, and causing organs such as the liver and kidneys to fail. Blisters of blood erupt below the skin. During these final stages of haemorrhagic fever clotting factors can become depleted (compare to the Andromeda Strain!), and blood vessels start to leak profusely, heavily tainting vomit and diarrhoea with blood. Shockingly, infected individuals literally bleed to death through every bodily orifice from their eyes to their ears! . It is during this final phase the virus is most infective due to the very high concentration of virus particles in the blood and body fluids. Undiagnosed patients can inadvertently spread the killer disease to family and healthcare workers. There is nothing more heart breaking than watching a distraught infected mother not being able to hug and console a crying child for fear of spreading the disease. This is an unforgiving virus and literally a teardrop can kill!
immunological correlates of protection or the genetic basis of resistance to Ebola. But sadly for the vast majority of infected individuals, the disease is devastating. The cunning virus “pirates” the immune system and incapacitates the anti-viral machinery of the immune cells by blocking the “interferon alarm” from sounding in target cells. The virus then triggers a “cytokine storm” triggering the release of potent inflammatory molecules into the circulatory system that wreak havoc throughout the body, and causing organs such as the liver and kidneys to fail. Blisters of blood erupt below the skin. During these final stages of haemorrhagic fever clotting factors can become depleted (compare to the Andromeda Strain!), and blood vessels start to leak profusely, heavily tainting vomit and diarrhoea with blood. Shockingly, infected individuals literally bleed to death through every bodily orifice from their eyes to their ears! . It is during this final phase the virus is most infective due to the very high concentration of virus particles in the blood and body fluids. Undiagnosed patients can inadvertently spread the killer disease to family and healthcare workers. There is nothing more heart breaking than watching a distraught infected mother not being able to hug and console a crying child for fear of spreading the disease. This is an unforgiving virus and literally a teardrop can kill!
The Achilles’ Heel of a Giant
As we learn more of the biology of the Ebola virus, scientists are uncovering ways to prevent or tame infections. The fatal power of this giant-sized virus is largely attributed to a single protein, known as the “spike-protein” and is the virus’s “magic wand”. Located on its surface, this protein is crucial for infecting cells, as well as manipulating the immune system. The shrewd virus cloaks and masks key domains of this protein using sugars, allowing it to evade the patrolling cells at the frontline of our immune systems. This spike-protein could also prove to be the virus’s Achilles’ heel, and insights into its structure will enable scientists to design new vaccines and drugs to target and inactivate vulnerable uncloaked regions in this essential protein.
 There are now prototype vaccines under development and undergoing fast-tracked human trials. A therapeutic cocktail of genetically engineered monoclonal antibodies known as ZMapp have been successfully used to neutralize the virus, providing passive immunity and protection against disease. The Ebola outbreak has also motivated me to return to my background in vaccine discovery and help contribute towards the development of vaccine against Ebola. To this end I have established a collaboration with my good friend Pietro Mastroeni (Cambridge) and Gary Kobinger (Canada). Gary is a pioneer in the field of viral haemorrhagic viruses, and senior author of the recent article in Nature describing the reversion and protective effects of the ZMapp antibody cocktail against Ebola. Our strategy will be to use synthetic biology to engineer an oral delivery platform for the vaccine, obviating the need for needles and syringes, or the requirement for refrigeration.
There are now prototype vaccines under development and undergoing fast-tracked human trials. A therapeutic cocktail of genetically engineered monoclonal antibodies known as ZMapp have been successfully used to neutralize the virus, providing passive immunity and protection against disease. The Ebola outbreak has also motivated me to return to my background in vaccine discovery and help contribute towards the development of vaccine against Ebola. To this end I have established a collaboration with my good friend Pietro Mastroeni (Cambridge) and Gary Kobinger (Canada). Gary is a pioneer in the field of viral haemorrhagic viruses, and senior author of the recent article in Nature describing the reversion and protective effects of the ZMapp antibody cocktail against Ebola. Our strategy will be to use synthetic biology to engineer an oral delivery platform for the vaccine, obviating the need for needles and syringes, or the requirement for refrigeration.
Global Health Security
Ebola has been considered a rare disease and consequently very little money has been invested in research or the development of therapeutics or vaccines to protect against disease. Big pharma have certainly steered clear for commercial reasons. The world governments, pharma, and international healthcare agencies need to co-operate and forge an alliance in readiness to prevent this and future outbreaks of infectious diseases from happening again. The international community has been slow in learning from the re-emergence of polio, cholera, or from the recent outbreaks caused by pathogens jumping from animals to humans. These include bird or swine flu (H7N9; H1N1), and the severe acute respiratory syndrome coronaviruses SARS and MERS. To keep the world safe from threats of infectious diseases, a major input of finance and resources from the national and international communities is required to provide essential support for research in microbiology and immunology, and establishing the necessary medical and management infrastructures essential in dealing with future episodes of Ebola. The present Ebola outbreak fortunately is now being brought under control. However in recent days there has been a British healthcare worker who has become infected in Sierra Leone, and her close contacts have also been flown back for treatment and monitoring in London and Newcastle. Fortunately, the Ebola outbreak will not become the global pandemic we all feared, and the UN predicts the outbreak will be over by the summer of 2015. However, big questions remain: Where does the virus go to in between these sporadic and unpredictable outbreaks? Does the virus fester away increasing in numbers in populations of fruit bats, waiting for the opportunity to jump across into primates? This is my great fear! The world should treat the present tidal wave of Ebola as a wake-up call. I hope I am wrong, but suspect complacency will prevail as Ebola begins to fade in our memories over the coming months. There is an apt quote from William Arthur Ward “The pessimist complains about the wind; the optimist expects it to change; the realist adjusts the sails”. The international community must become “realists”, and adjust their responses to act now and fulfill all their promises of funding and resources! Otherwise, this could provide a tragic opportunity for a microbiological tsunami to hit the shores of every country!