Remember, remember the 5th of November, gunpowder, treason and plot! We see no reason why the science of fire should ever be forgot!
For this bonfire night, we are looking into the gravity defying properties of water using fire!
Step 1
Pour the water into your container and add the food colouring to colour the water to whatever colour you like, we chose blue.
Step 2
Place the candle in the middle of the water but make sure the wick and wax of your candle stays dry.
Step 3
Get an adult to help you light the candle and make sure the wick is burning for about 20 seconds before moving onto step 4.

Step 4
Place your glass/plastic cup over the candle, this will push all the water away from the candle

Step 5
Wait for a few moments and watch the candle go out and the water rise on the inside of the cup!

The science!
First of all, why does the candle go out?
Fire needs three things to burn; oxygen, fuel and heat. These three things make up the fire triangle which you can see below.
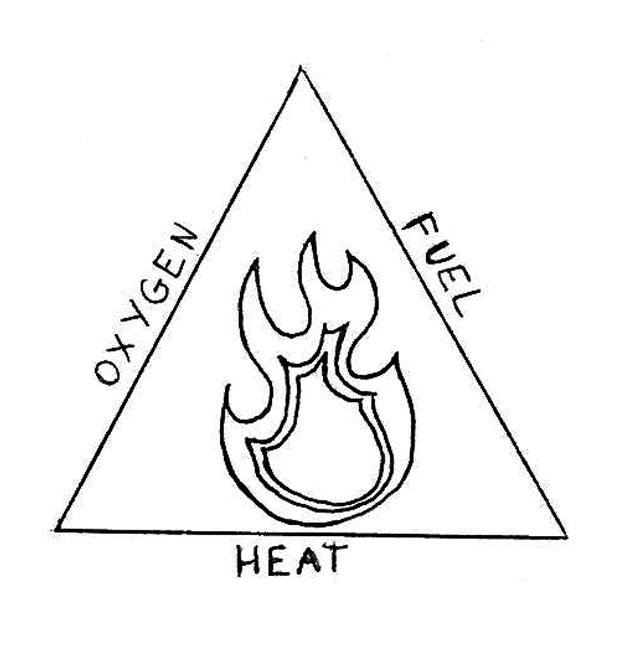
If one of them is taken away, the fire is put out. By putting the cup over the candle, the oxygen is taken away from the fire so it goes out!
But… it doesn’t go out straight away. This is because there is still some oxygen trapped inside the cup but once the fire has used up all the oxygen there is none left so the candle goes out.
So, why does the water in the cup rise after the flame goes out? When the candle is lit, the particles in the air take in some of the heat from the flame and get hotter. When the particles get hotter, they have more energy so move faster and this increases the pressure inside the cup.
After the flame has gone out, the particles cool down and move more slowly and this decreases the pressure in the cup. The pressure outside the cup is then higher than inside the cup so the water is pushed inside the cup until the pressure outside the cup is the same as the pressure inside the cup.