For this week’s experiment you will need to raid your fridge and kitchen cupboards to get some milk, food colouring and washing up liquid.
Pour some milk into a dish or bowl, this works better with full fat milk (we’ll tell you why later!). Add small drops of your food colouring wherever you like in the milk.
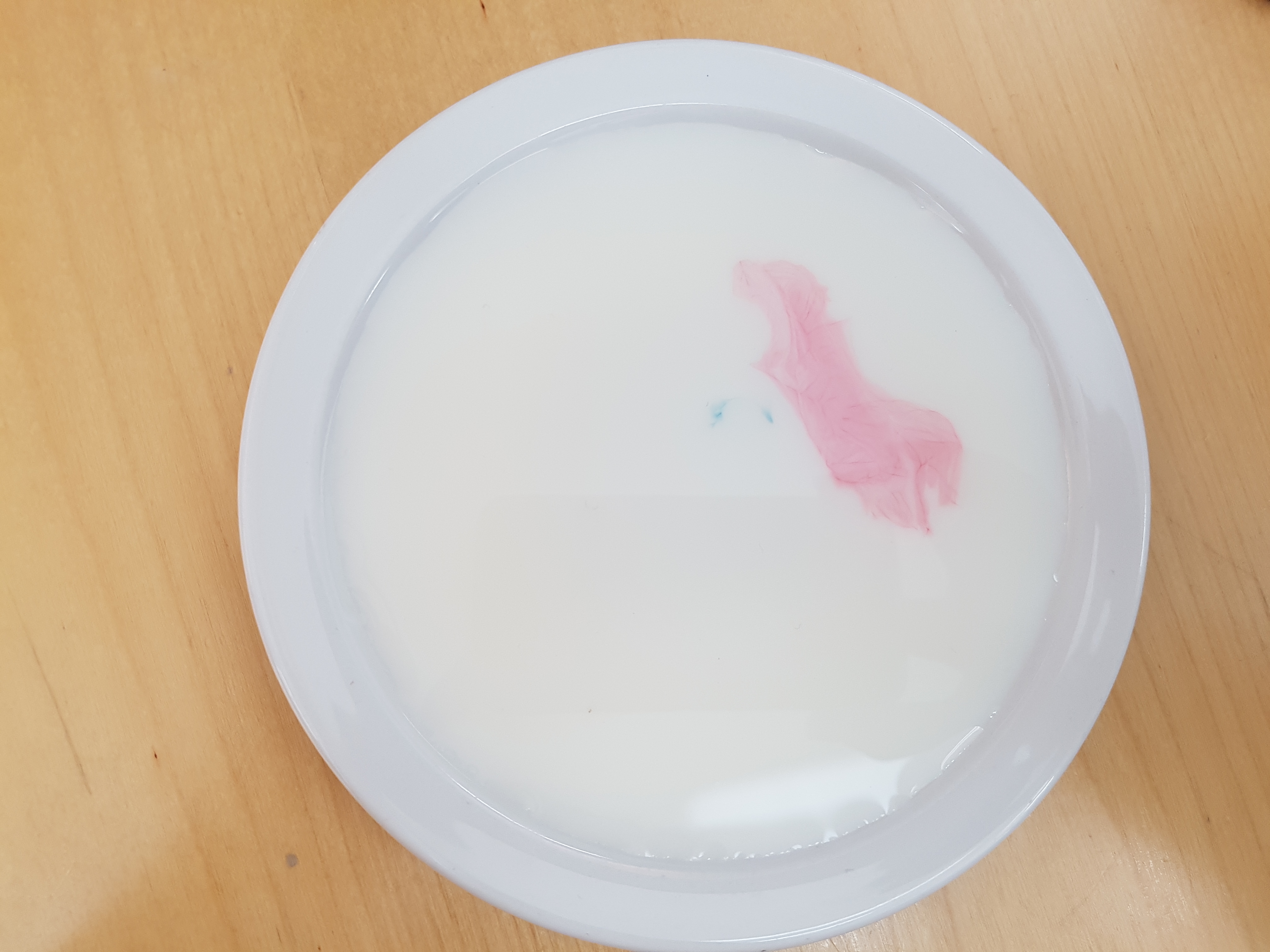
Get some washing up liquid on the end of a spoon or cotton bud and gently tap the spots of food colouring with it. 
The food colouring should burst out into colourful stars and wavy shapes. This happens because the washing up liquid molecules have a hydrophobic tail, these means that they don’t like water so try to get away from it by seeking fat molecules. The milk (especially if it is full fat milk) contains lots of fat molecules. So the washing up liquid moves around in the milk seeking out this fat and takes the food colouring along with it, creating these funky patterns.
This is why we use washing up liquid to clean our dishes. The hydrophobic, fat-loving parts cling to grease and fat. The head of the washing up molecules are hydrophilic, meaning they love water. The heads cling to the water and the tails cling to the grease, this pulls the grease and dirt from your plates and washes them away with the water, giving you sparkly clean dishes.


 Try making different flavours of ice cream by swapping the vanilla extract for strawberry or mint extract or even cocoa powder for chocolate ice cream. You could also try adding chocolate chips.
Try making different flavours of ice cream by swapping the vanilla extract for strawberry or mint extract or even cocoa powder for chocolate ice cream. You could also try adding chocolate chips.