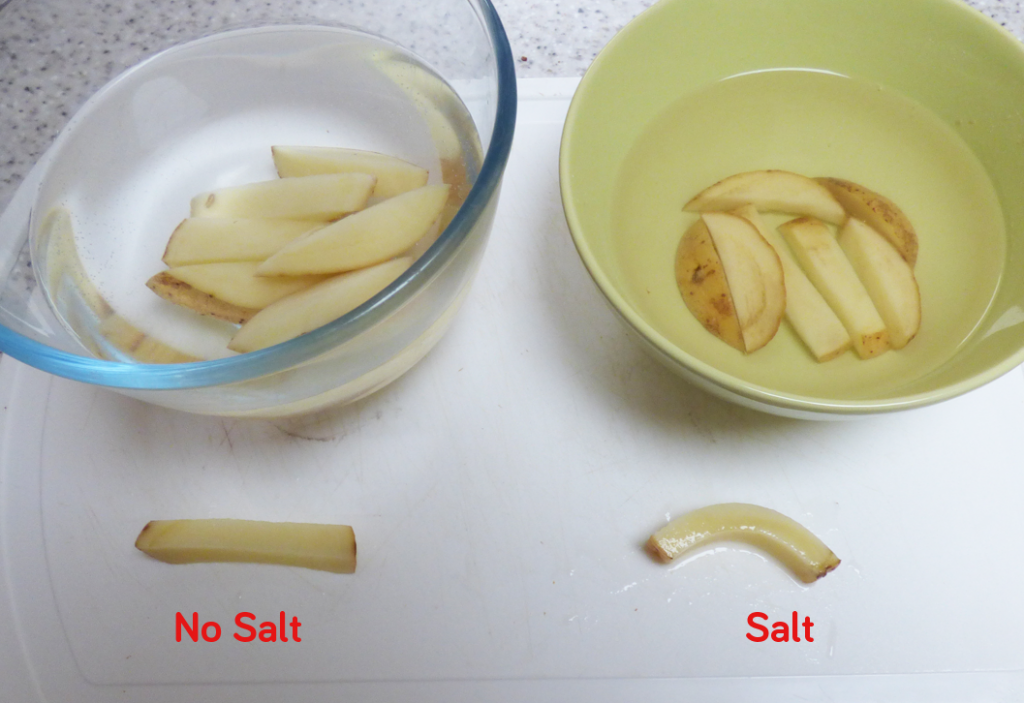
Today we are looking at the science behind curly potato fries. First, let’s talk about how we make them.
- Carefully chop up a potato into straight thick chips.

- Boil around 250ml of water and stir salt into this water until no more salt will dissolve.
- Fill a bowl with tap water and place half of your chips into this bowl.
- When the salty water has cooled pour it into another bowl and add the rest of your chips to this.

- Leave both bowls of chips out overnight.
- The next day you should have one bowl of chips that are still hard and straight and the other bowl (with salty water in) will be full of chips that are more flexible, that you can shape into curls.
The Science
The addition of salt to the water allows you to make curly fries due to osmosis. Osmosis is the movement of water from an area that has few molecules in the water to an area that has more molecules in it to try to even things out and create a balance.
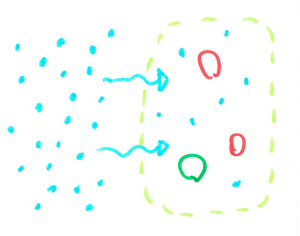
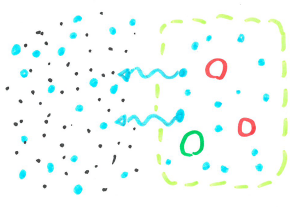
Plants like our potato here are made up of millions of cells that have a cell membrane around its edge which allows some things in and not others. Water can easily flow through this but the salt we dissolved in it can’t. Cells are filled with lots of little molecules so water usually flows into the cells and fills them to dilute the liquid. But when we have lots of salt in the water, there are more particles in the water outside of the potato cells than inside so the water leaves the cells.
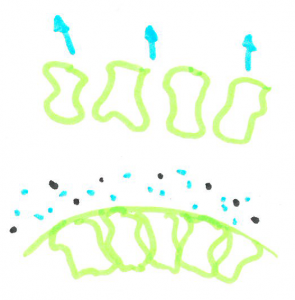 When cells are filled with water they are quite rigid and packed closely together making a fairly sturdy chip. When the cells are dehydrated, they are smaller leaving space between cells, allowing the chip to bend without snapping.
When cells are filled with water they are quite rigid and packed closely together making a fairly sturdy chip. When the cells are dehydrated, they are smaller leaving space between cells, allowing the chip to bend without snapping.
Osmosis is used in all plants – not just when you cut them up and put them in a bowl of water! Plants use osmosis in their roots to allow water to move from the soil into their roots.


 Try making different flavours of ice cream by swapping the vanilla extract for strawberry or mint extract or even cocoa powder for chocolate ice cream. You could also try adding chocolate chips.
Try making different flavours of ice cream by swapping the vanilla extract for strawberry or mint extract or even cocoa powder for chocolate ice cream. You could also try adding chocolate chips.