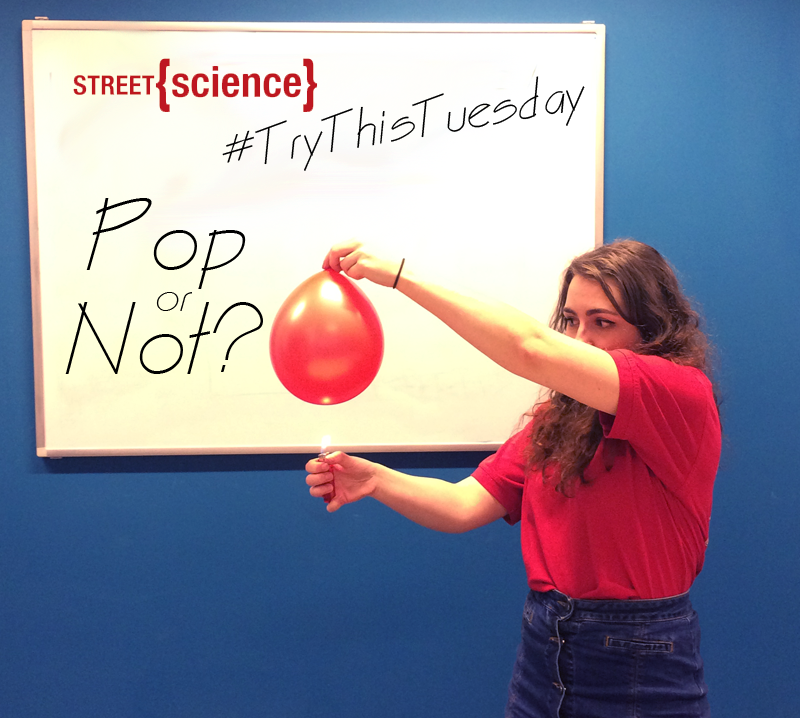
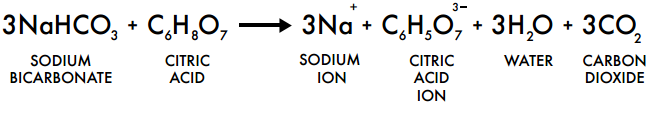You will need: large tall glass, bicarbonate of soda (baking soda), vinegar, a candle and some matches
 1. Add 4 teaspoons of bicarbonate of soda to the glass
1. Add 4 teaspoons of bicarbonate of soda to the glass
2. Pour in roughly 150ml of vinegar, the mixture will fizz.

3. Light the candle.
4. Once the mixture has stopped fizzing, pick up the glass. Without pouring out the vinegar, gently tip the glass from a few centimeters above the candle. Imagine that there is an invisible liquid inside above the mixture. The candle will go out!

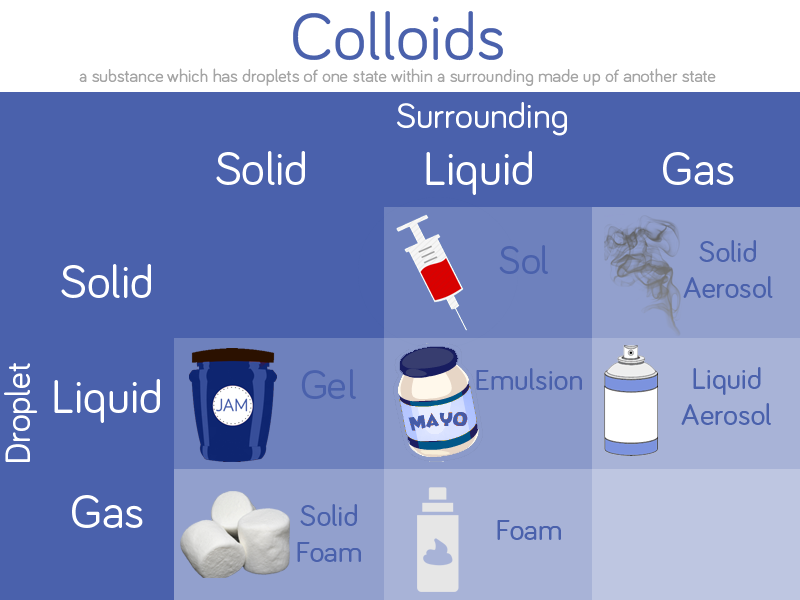
The Science
You have produced a gas, carbon dioxide, by mixing the bicarbonate of soda with vinegar (also known as acetic acid). Bicarbonate of soda contains carbon dioxide, but it is attached to other molecules. When you mix it with vinegar the bicarbonate breaks down and releases carbon dioxide as a gas.
The following reaction takes place:
bicarbonate of soda + vinegar → sodium acetate + water + carbon dioxide
NaHCO3 + HC2H3O2 → NaC2H3O2 + H2O + CO2
Carbon dioxide is heavier than air so stays in the glass until you tip it over the candle. When you pour carbon dioxide on a candle it stops the flow of oxygen which is needed for a flame to burn, and the candle is extinguished.
Real fire extinguishers also use carbon dioxide to put out fires, it is compressed (squashed) into cylinders and sprayed at fires.