During our time as STEM Ambassadors, we’ve visited several beaches together. From Newcastle in Northern Ireland to Clear Water Bay in Hong Kong and even beaches closer to home in Whitley Bay and Tynemouth, we always ended up skipping rocks somewhere!
But how do we do it!? Why don’t the rocks just fall into the water?

The key is to get a nice flat rock and throw it quickly at the right angle. The large surface area allows the stone to bounce off the water’s surface.
You need to throw it fairly hard to give it enough speed to gain momentum before it hits the water. When the rock hits the surface of the water it pushes the water down whilst the water pushes the rock up. If the force pushing the stone up from the water is greater than or balances the weight of the stone then it will bounce on for another skip rather than sinking. This is why it helps to have a nice small stone.

It is also important to get the right velocity. Velocity is the speed of something in a give direction. So we have the speed covered, now for the direction. Scientists have discovered that the optimal angle at which the stone should hit the water should be around 20 degrees. As you probably won’t be able to measure this on a causal day trip to the beach, just aim to throw the stone sideways rather than up or down.
Hopefully you’ll manage more than my measly two skips. Try beating the world record of 88 skips in a row!



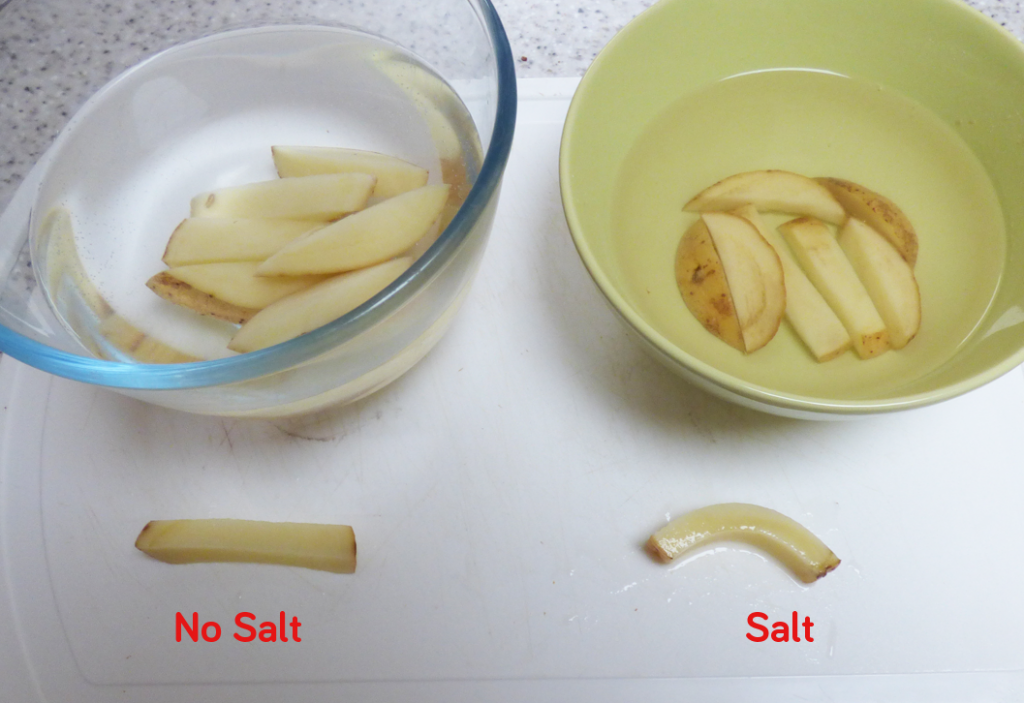
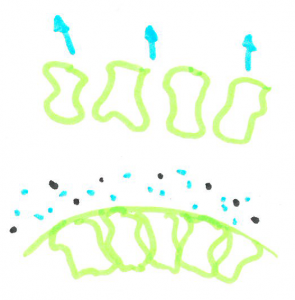 When cells are filled with water they are quite rigid and packed closely together making a fairly sturdy chip. When the cells are dehydrated, they are smaller leaving space between cells, allowing the chip to bend without snapping.
When cells are filled with water they are quite rigid and packed closely together making a fairly sturdy chip. When the cells are dehydrated, they are smaller leaving space between cells, allowing the chip to bend without snapping.

 Try making different flavours of ice cream by swapping the vanilla extract for strawberry or mint extract or even cocoa powder for chocolate ice cream. You could also try adding chocolate chips.
Try making different flavours of ice cream by swapping the vanilla extract for strawberry or mint extract or even cocoa powder for chocolate ice cream. You could also try adding chocolate chips.