As Easter is coming up we’re treating you to four experiments instead of one this week! And a little video showing Ellie and Clare demonstrating each of them (or at least attempting to!)
1. Hard-boiled Egg Test
Our first eggsperiment requires a hard boiled egg so we’re going to show you how you can test if an egg is hard boiled or not.
Lay a hard-boiled and a normal egg flat on their sides and spin them. Put your finger on the eggs to stop spinning them and then let go. The one that starts spinning again is the raw egg.
This is all due to momentum. When you spin the eggs you spin their insides too. In the hard boiled egg, the insides are fixed to the shell so it behaves as you would expect. In the raw egg the insides continue to spin after you’ve stopped the shell. When you let go, the momentum of the spinning yolk carries the shell and the whole egg starts spinning again.
2. Egg in a Bottle
Now we know which is our hardboiled egg, we need to peel it for next experiment. This one requires a glass bottle, a match and of course, the egg.
If we place the egg on top of the bottle it doesn’t look like it’s going to fit in. But if we light the match and drop it into the bottle then after a second place the egg on top, the egg will squeeze into the bottle.
The match heats up the air in the bottle, causing it to expand slightly. The egg creates a seal so more air cannot enter. As the air cools inside the bottle it decreases the pressure and forces the egg into the bottle.
3. Naked Bouncy Egg
To make a naked bouncy egg you will need an egg, white vinegar and a beaker. Place your egg in the beaker and pour in enough vinegar to cover the egg. Leave you egg here overnight.
The egg shell is made mostly of calcium carbonate, this reacts with the acid in the vinegar and dissolves to leave a naked egg. It also produces carbon dioxide gas, so as your egg is soaking you may see little bubbles of CO2 forming around it.
After a day, carefully remove you egg from the vinegar and wipe away any remaining shell. You should see that it’s now quite rubbery and bouncy as well.
4. Bouncy Elastic Egg Drop
For this final eggsperiement you can use your bouncy naked eggs but we’re cheating a little bit and using rubber eggs. For this you will need to take two rubber eggs and join them together with a piece of elastic or a string of elastic bands.
When you hold your eggs next to one another and let go they both hit the floor at the same time as you might expect. But what do you think will happen if we just hold the top egg and let the other hang below it, which will reach the floor first when we let go?
Gravitational acceleration is the same no matter the weight or mass of an object, but when we add elastic between the eggs, this adds an extra force that speeds up the drop of the higher up egg as the elastic pulls them together.
















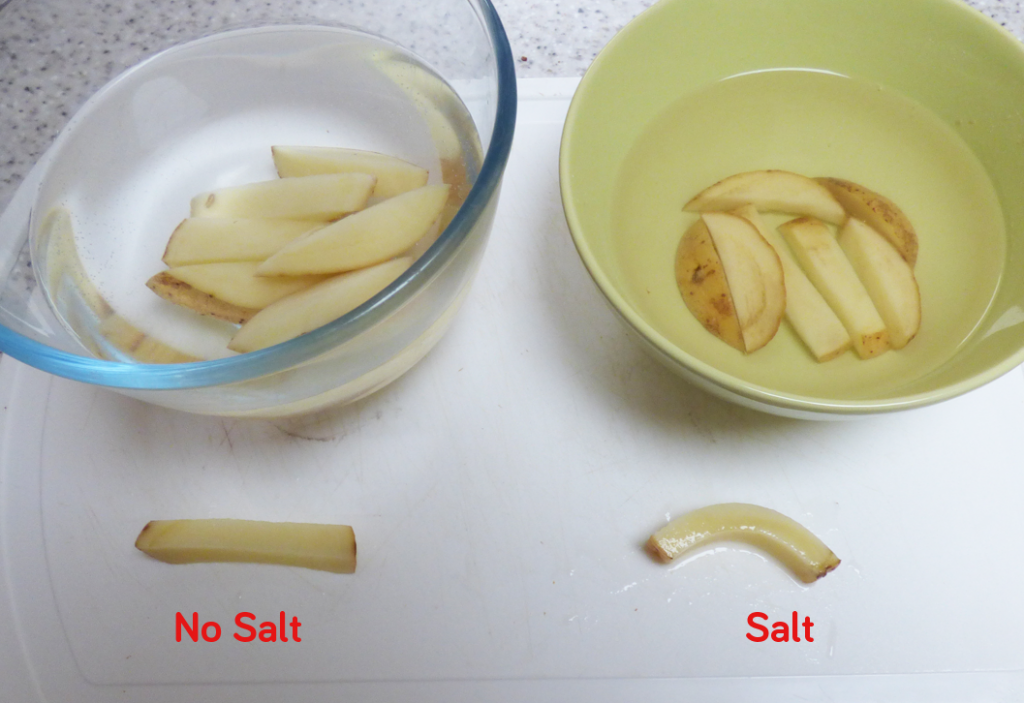
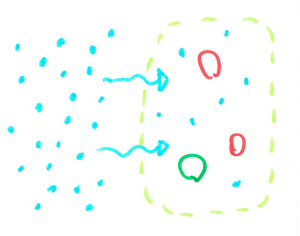
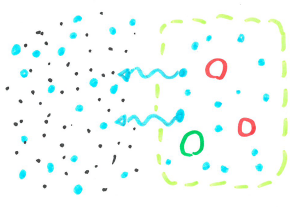
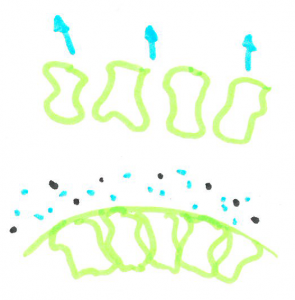 When cells are filled with water they are quite rigid and packed closely together making a fairly sturdy chip. When the cells are dehydrated, they are smaller leaving space between cells, allowing the chip to bend without snapping.
When cells are filled with water they are quite rigid and packed closely together making a fairly sturdy chip. When the cells are dehydrated, they are smaller leaving space between cells, allowing the chip to bend without snapping.

 Try making different flavours of ice cream by swapping the vanilla extract for strawberry or mint extract or even cocoa powder for chocolate ice cream. You could also try adding chocolate chips.
Try making different flavours of ice cream by swapping the vanilla extract for strawberry or mint extract or even cocoa powder for chocolate ice cream. You could also try adding chocolate chips.



